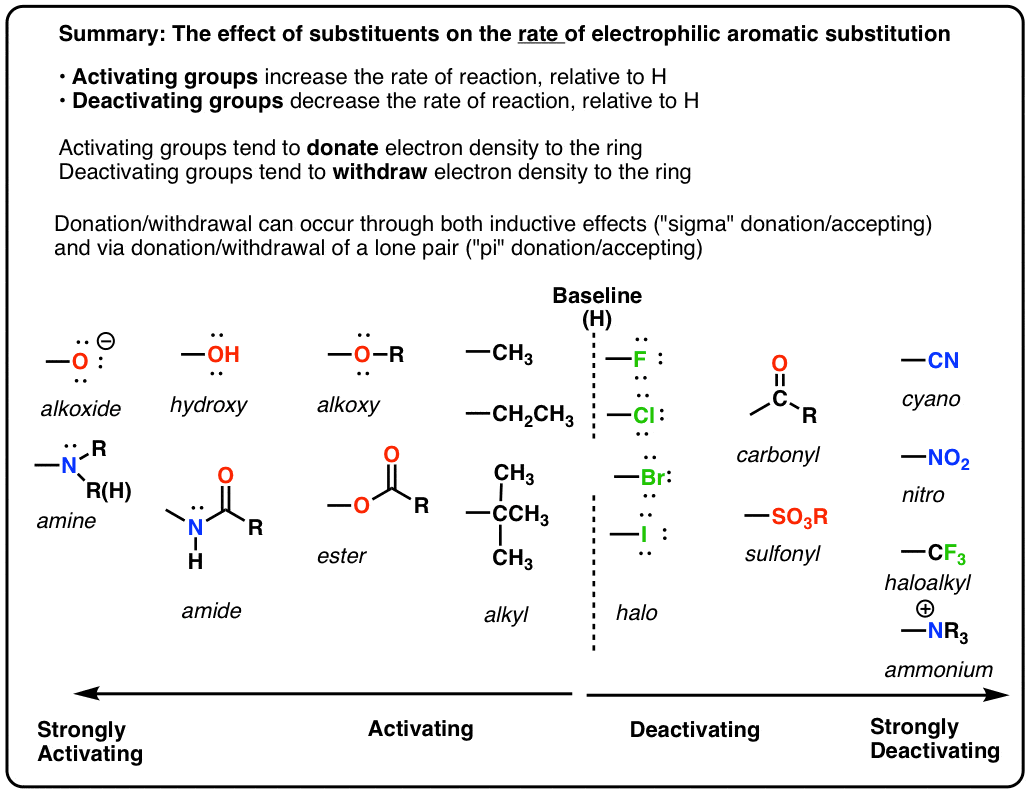
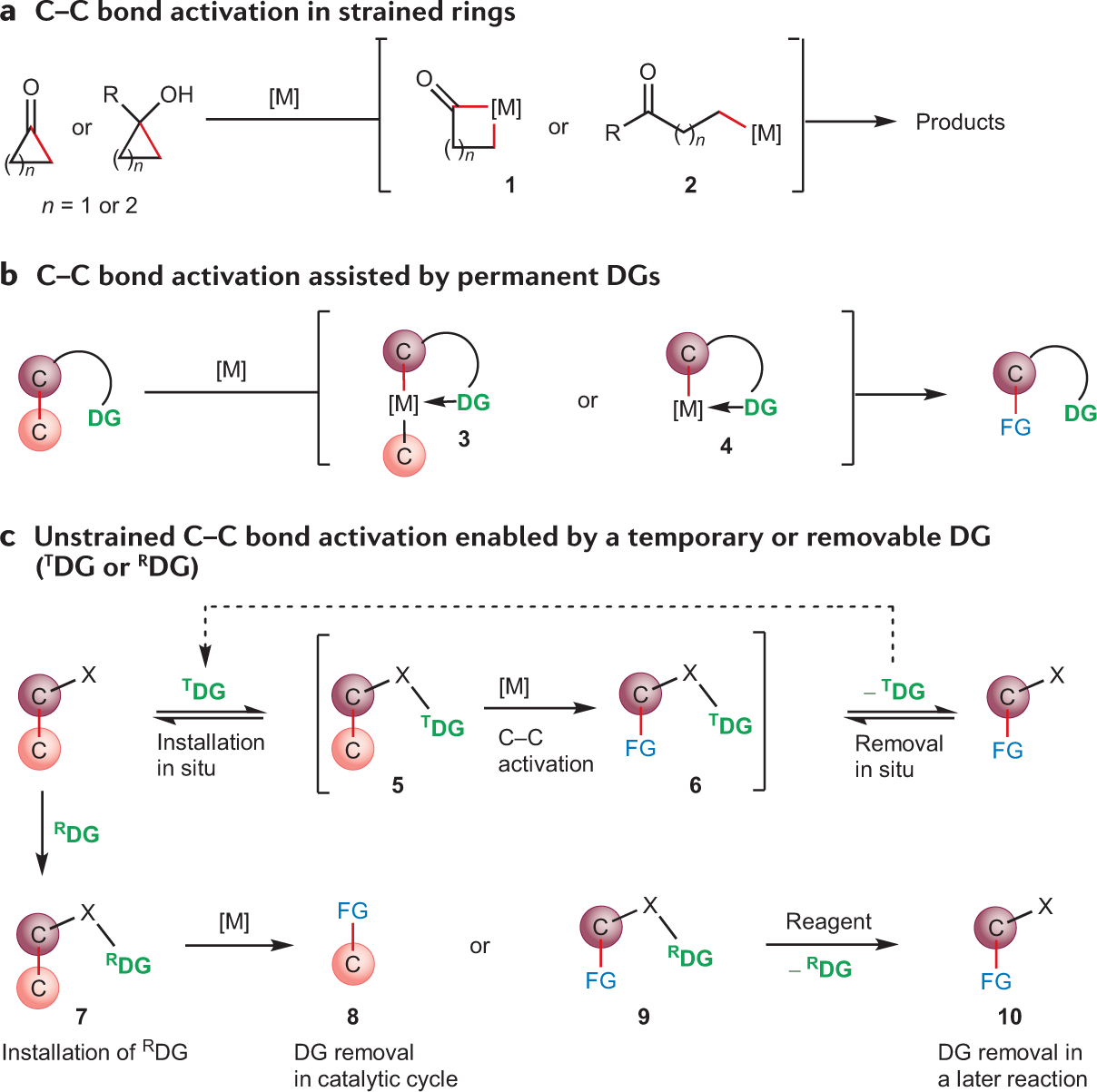
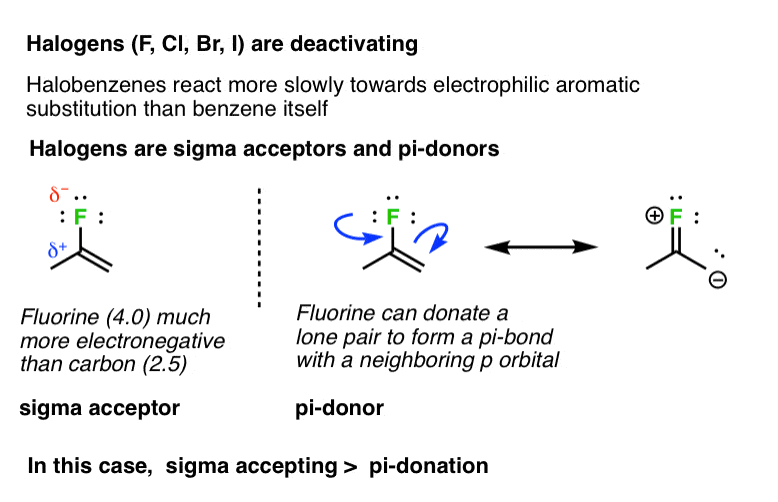
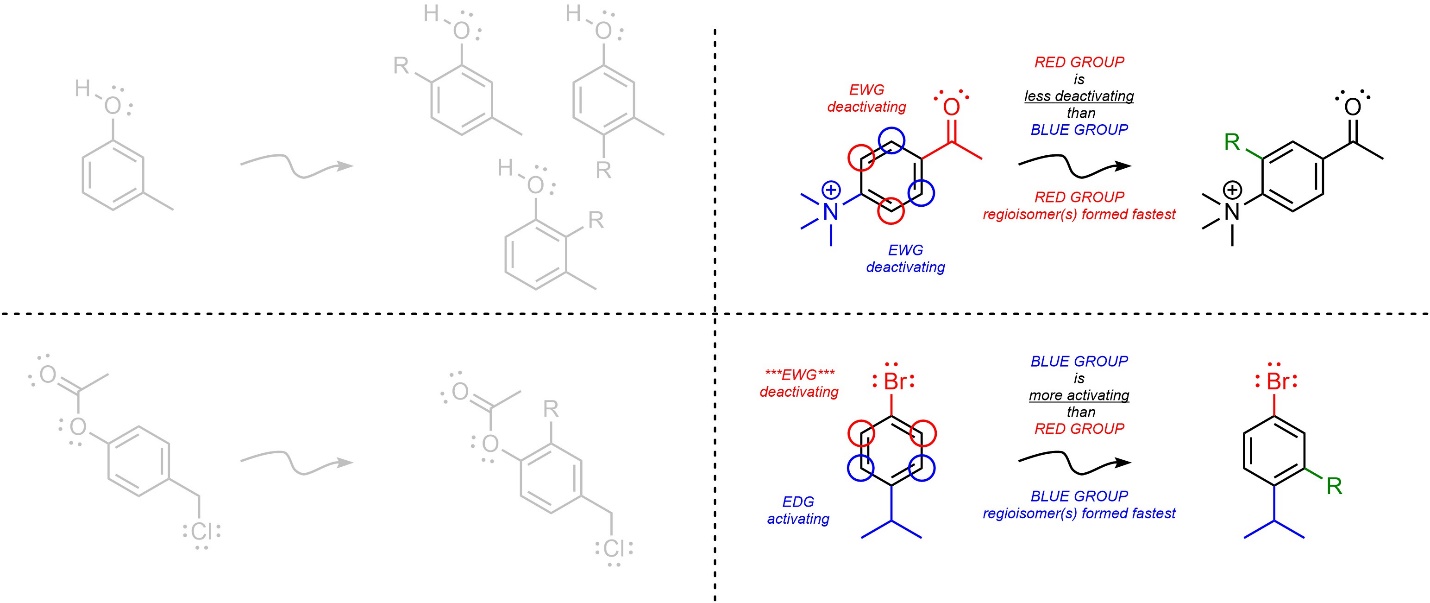
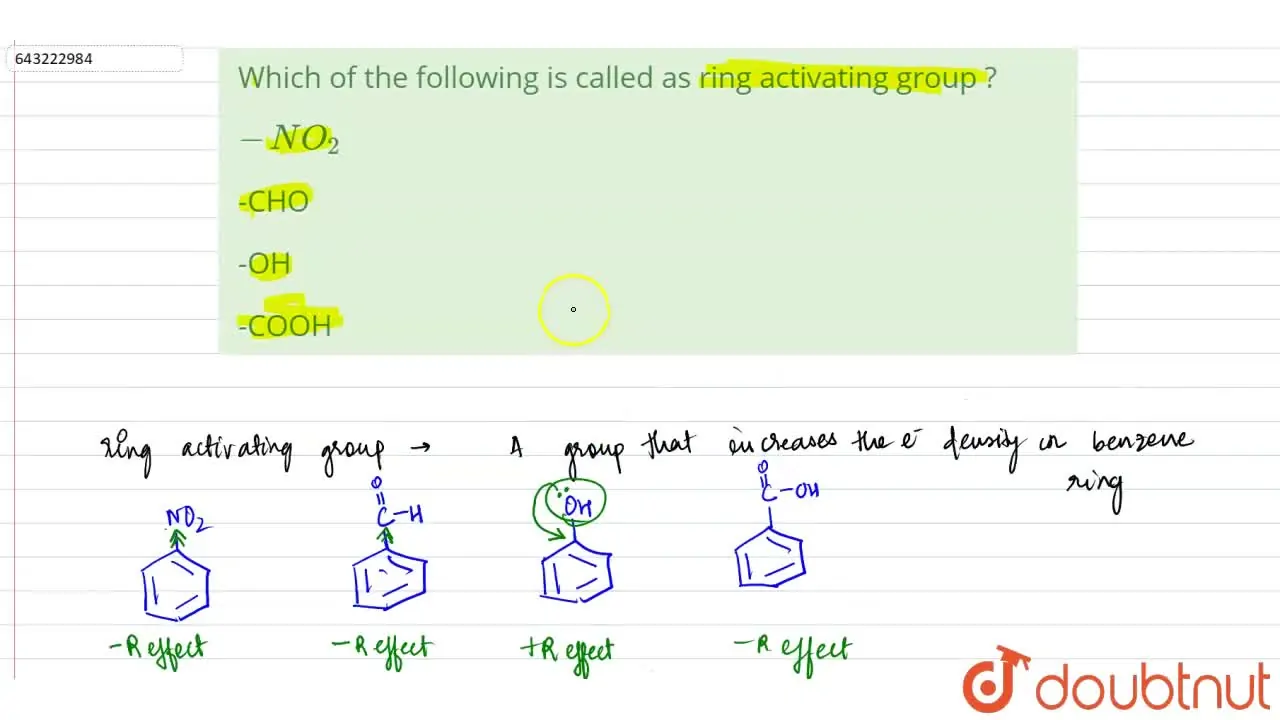
Aggregate more than 160 ring activating groups best
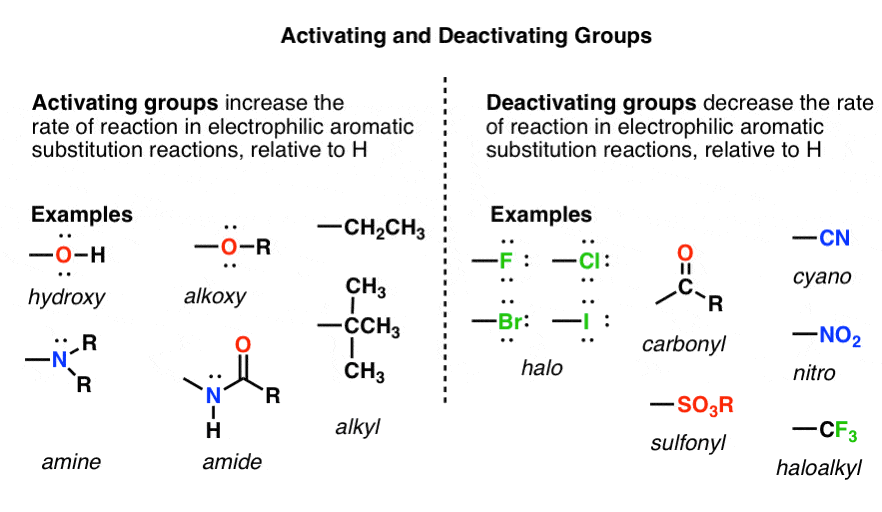
Details images of ring activating groups by website dienmayquynhon.com.vn compilation. Solved a) Identify the number of activating and deactivating | Chegg.com. SOLVED: 1. Consider the following molecule: a) Do you expect the group attached to the benzene ring ” to be activating O deactivating, and why? Provide a brief justification for your response,. Acidity, Effect of Substituents on Acidity and Important Reactions of Benzoic Acid : Pharmaguideline. Board \& Competitive Exams (Level- 52. The most deactivating group for el… PG Organic Unit – IV | PDF
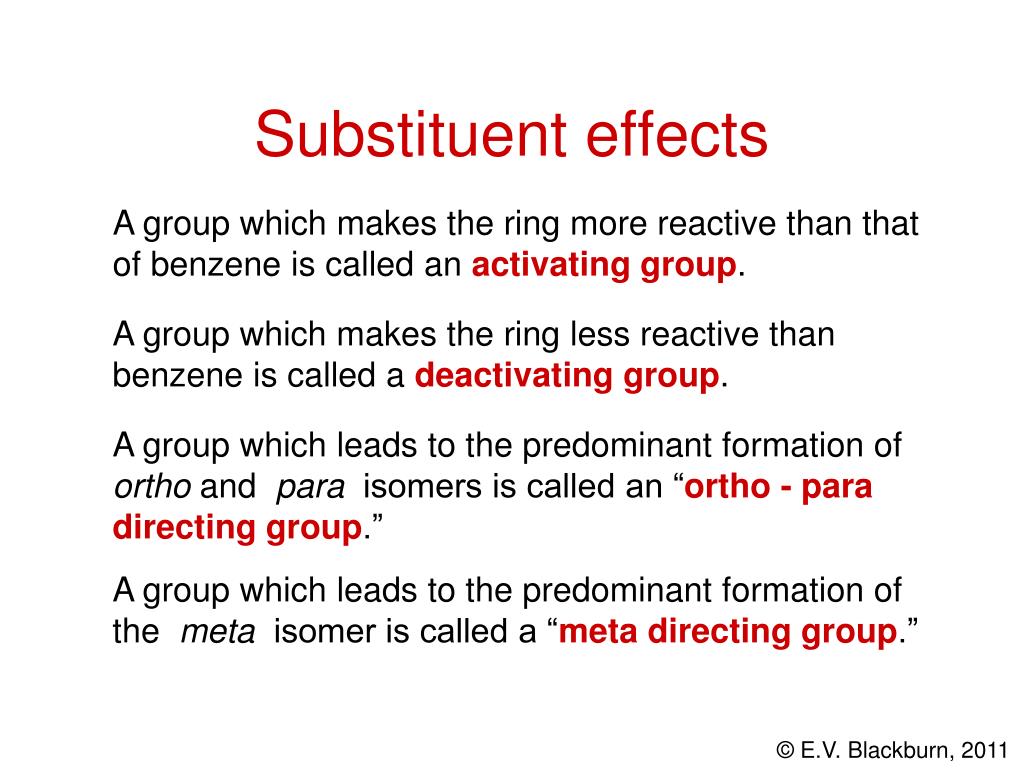
 5. Benzene and Aromaticity Aromatic Compounds The term “Aromatic” is used to refer to the class of compounds structurally related to Benzene. The first. – ppt download – #1
5. Benzene and Aromaticity Aromatic Compounds The term “Aromatic” is used to refer to the class of compounds structurally related to Benzene. The first. – ppt download – #1
- activating groups examples
- electron rich aromatic rings
- activating group order
 Why CH3 group is Ortho and para directing in nature in Toulene ? – The Unconditional Guru – #2
Why CH3 group is Ortho and para directing in nature in Toulene ? – The Unconditional Guru – #2
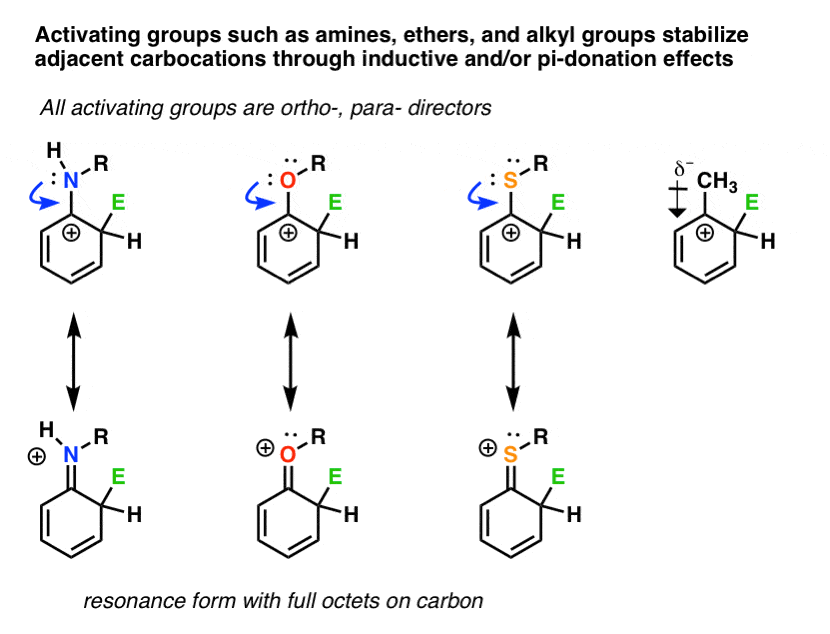 SOLVED: Substituents on an aromatic ring can have several effects on electrophilic aromatic substitution reactions. Substituents can activate Or deactivate the ring to substitution, donate or withdraw electrons inductively, donate Or withdraw – #3
SOLVED: Substituents on an aromatic ring can have several effects on electrophilic aromatic substitution reactions. Substituents can activate Or deactivate the ring to substitution, donate or withdraw electrons inductively, donate Or withdraw – #3
- meta directing groups list
- aromatic electron withdrawing groups
- electron donating and withdrawing groups mcat
 Question #1ad34 + Example – #4
Question #1ad34 + Example – #4
 AROMATIC ELECTROPHILIC SUBSTITUTION – #5
AROMATIC ELECTROPHILIC SUBSTITUTION – #5
 Benzyl group – Wikipedia – #6
Benzyl group – Wikipedia – #6
 organic chemistry – Why carbonyl groups are strong benzene deactivating group for electrophillic aromatic substitution? – Chemistry Stack Exchange – #7
organic chemistry – Why carbonyl groups are strong benzene deactivating group for electrophillic aromatic substitution? – Chemistry Stack Exchange – #7
 20 Therapy Tools to Manage Anxiety & Distress – #8
20 Therapy Tools to Manage Anxiety & Distress – #8
 PDF) Review : General Principles in Chemistry – #9
PDF) Review : General Principles in Chemistry – #9
 🖩 CPCA Calculation Tool (excel-based) -Updated – Limits of Nitrosamines – Nitrosamines Exchange – #10
🖩 CPCA Calculation Tool (excel-based) -Updated – Limits of Nitrosamines – Nitrosamines Exchange – #10
 orientation of products substituent in monosubstituted benzene derivatives ortho meta para substitution resonance structures hybrid methylbenzene chlorobenzene phenol nitrobenzene benzoic acid effect on synthesis routes advanced A level organic … – #11
orientation of products substituent in monosubstituted benzene derivatives ortho meta para substitution resonance structures hybrid methylbenzene chlorobenzene phenol nitrobenzene benzoic acid effect on synthesis routes advanced A level organic … – #11
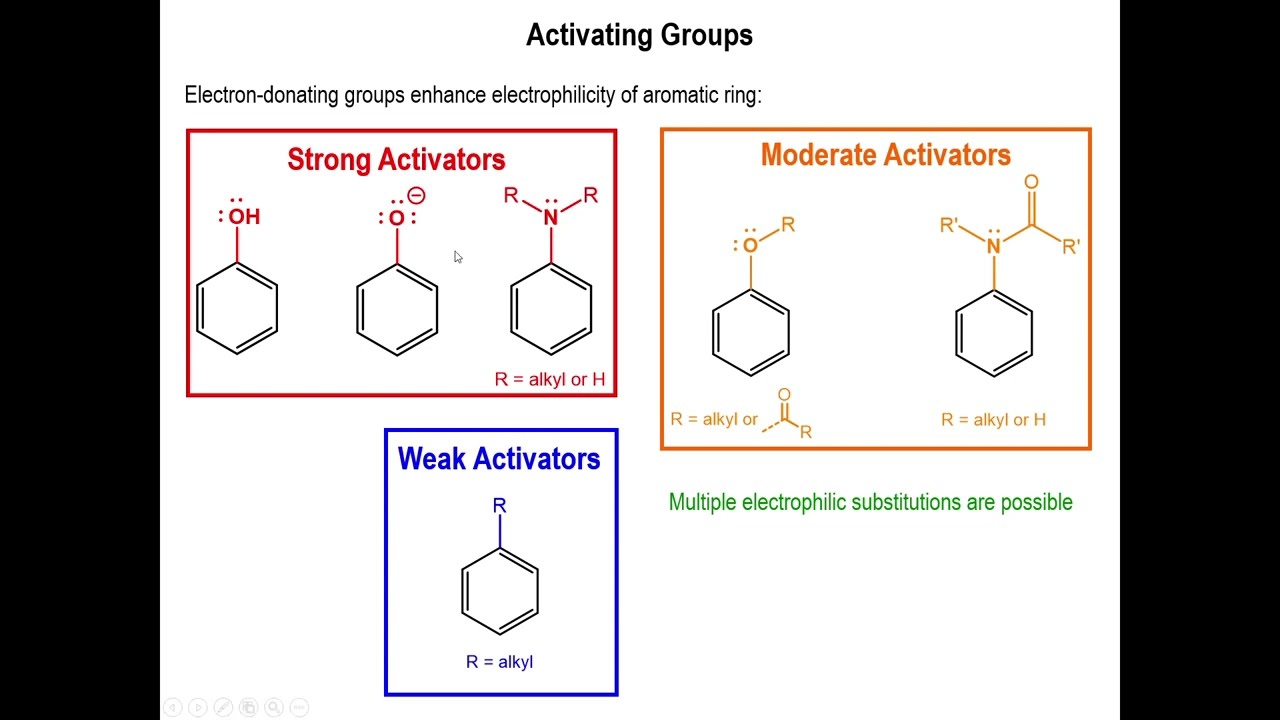 Sheet 1 Eas | PDF | Benzene | Organic Reactions – #12
Sheet 1 Eas | PDF | Benzene | Organic Reactions – #12
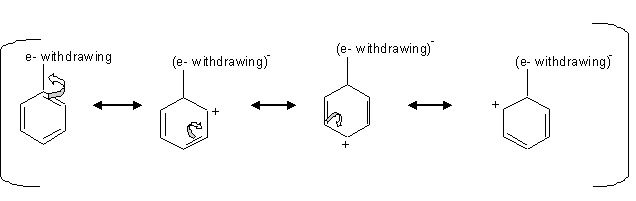 Chapter 8 Aromaticity Reactions of Benzene. Aromatic compounds undergo distinctive reactions which set them apart from other functional groups. They. – ppt download – #13
Chapter 8 Aromaticity Reactions of Benzene. Aromatic compounds undergo distinctive reactions which set them apart from other functional groups. They. – ppt download – #13
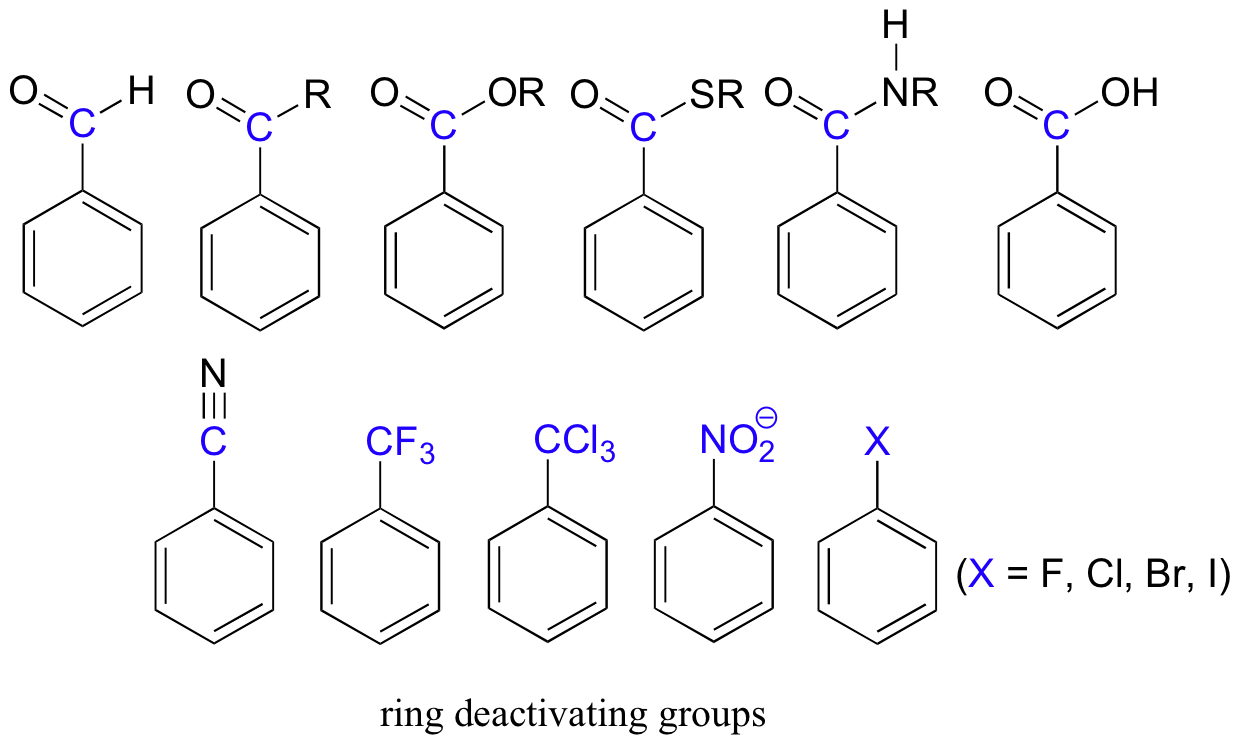 16.S: Chemistry of Benzene – Electrophilic Aromatic Substitution (Summary) – Chemistry LibreTexts – #14
16.S: Chemistry of Benzene – Electrophilic Aromatic Substitution (Summary) – Chemistry LibreTexts – #14
 Which of the following is true about following when attached on benzene. ama C CI is activating group Cl is activating group SERIES SAREBBE FREE -O-C-CH, is deactivating group -CH is activating – #15
Which of the following is true about following when attached on benzene. ama C CI is activating group Cl is activating group SERIES SAREBBE FREE -O-C-CH, is deactivating group -CH is activating – #15
 The product formed is:\n \n \n \n \n A.\n \n \n \n \n B.\n \n \n \n \n C. Pyridine acts as deactivating and o and p – directingD. Pyridine acts as – #16
The product formed is:\n \n \n \n \n A.\n \n \n \n \n B.\n \n \n \n \n C. Pyridine acts as deactivating and o and p – directingD. Pyridine acts as – #16
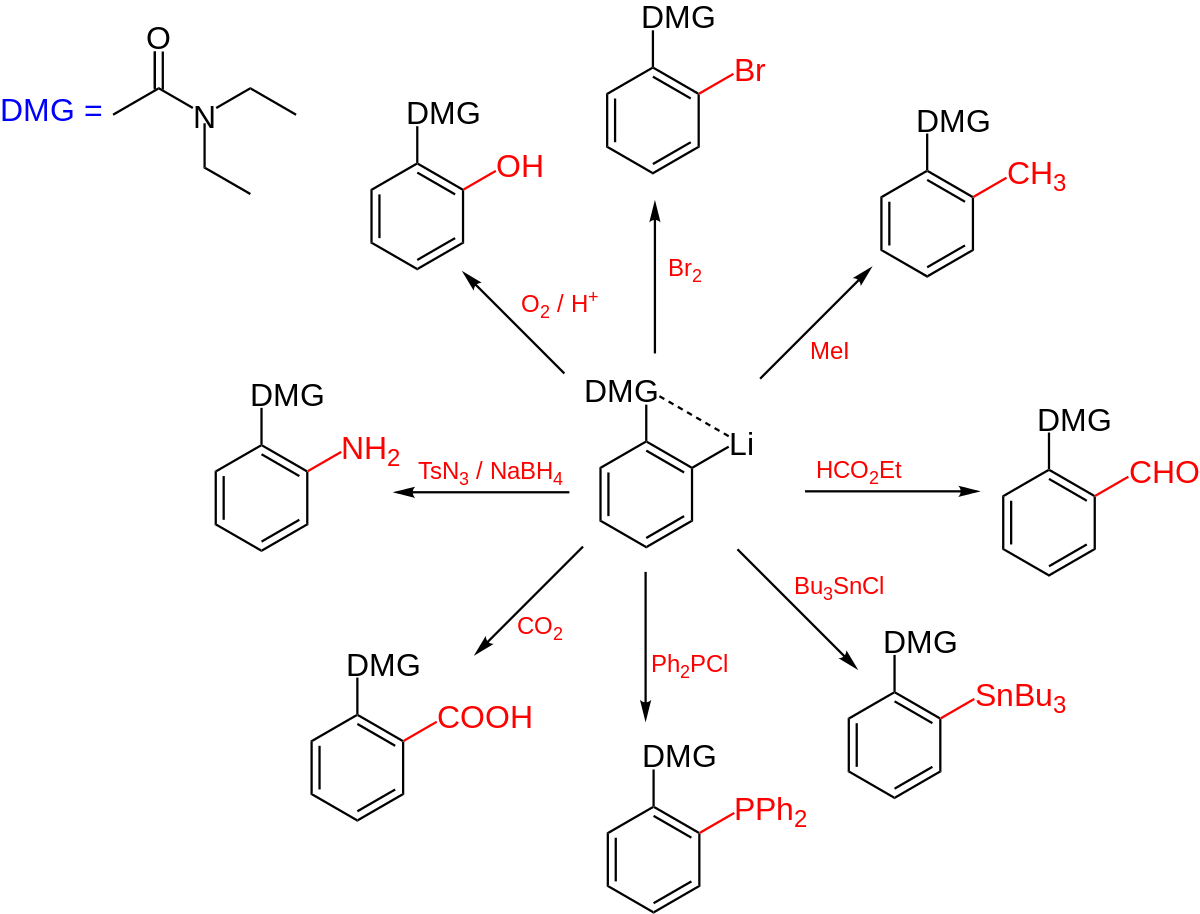 SOLVED: Indicate whether each group on a benzene ring, listed below, directs an incoming electrophile (E+, for example, nitronium ion, *NO2) ortho and para (o,p) or meta (m): (12 points): CH2CH2CH3 CF3 – #17
SOLVED: Indicate whether each group on a benzene ring, listed below, directs an incoming electrophile (E+, for example, nitronium ion, *NO2) ortho and para (o,p) or meta (m): (12 points): CH2CH2CH3 CF3 – #17
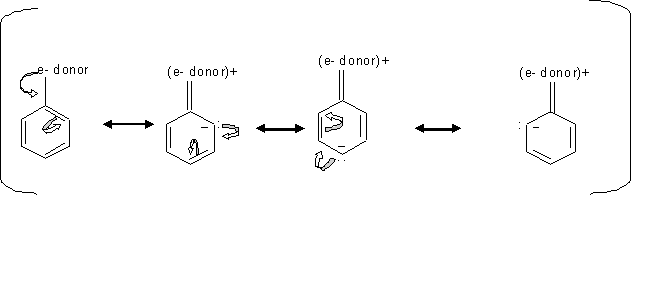 Reactions of Aromatic Compounds – #18
Reactions of Aromatic Compounds – #18
![Solved] Predict whether the following substituents on the benzene ring are... | Course Hero Solved] Predict whether the following substituents on the benzene ring are... | Course Hero](https://cdn.masterorganicchemistry.com/wp-content/uploads/2019/12/5-table-of-activating-and-deactivating-groups-strongly-activating-moderate-mildly-activating-mildly-deactivating-strongly-deactivating.gif) Solved] Predict whether the following substituents on the benzene ring are… | Course Hero – #19
Solved] Predict whether the following substituents on the benzene ring are… | Course Hero – #19
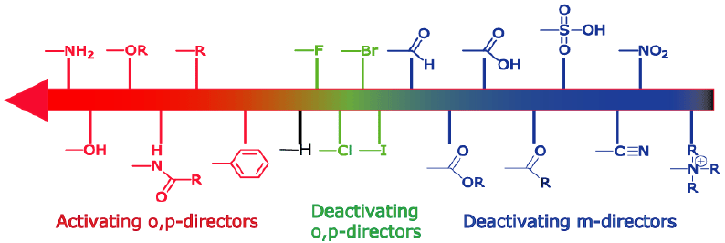 Selective deoxygenative alkylation of alcohols via photocatalytic domino radical fragmentations | Nature Communications – #20
Selective deoxygenative alkylation of alcohols via photocatalytic domino radical fragmentations | Nature Communications – #20
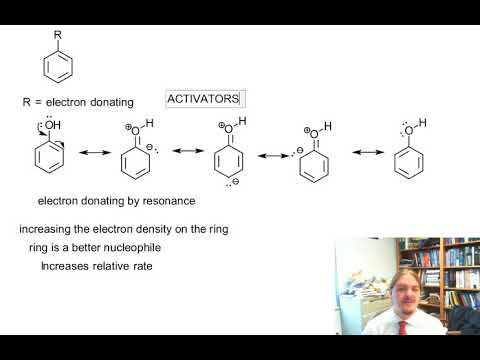
 Ring activating and deactivating groups – YouTube – #21
Ring activating and deactivating groups – YouTube – #21
 Chapter 9 Second Half. Electrophilic aromatic substitution electrophile (E + ) reacts with an aromatic ring and substitutes for one of the hydrogens The. – ppt download – #22
Chapter 9 Second Half. Electrophilic aromatic substitution electrophile (E + ) reacts with an aromatic ring and substitutes for one of the hydrogens The. – ppt download – #22
 EAS Ortho Para & Meta Directors and Activating & Deactivating Groups – Chad’s Prep® – #23
EAS Ortho Para & Meta Directors and Activating & Deactivating Groups – Chad’s Prep® – #23
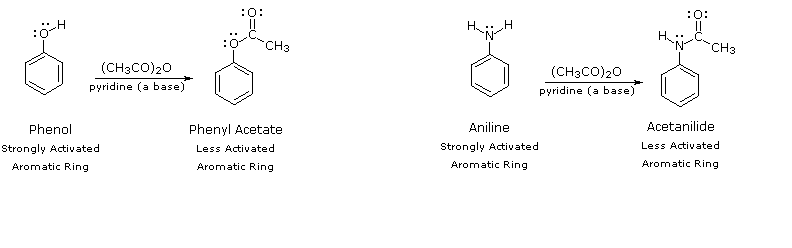
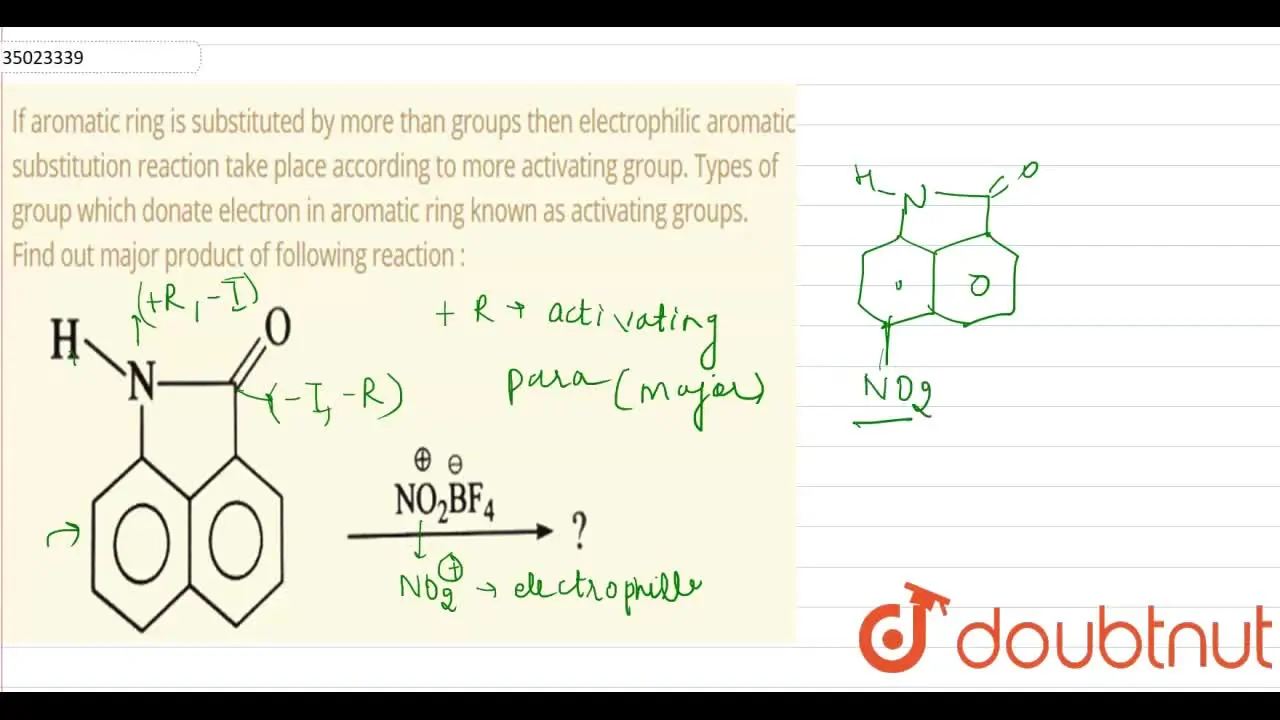 Solved 5. Explain why the-NHCOCH3 in acetanilide is only | Chegg.com – #24
Solved 5. Explain why the-NHCOCH3 in acetanilide is only | Chegg.com – #24
 PPT – Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution PowerPoint Presentation – ID:2772837 – #25
PPT – Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution PowerPoint Presentation – ID:2772837 – #25
![Solved] Given that increasing electron density in an aromatic ring makes it... | Course Hero Solved] Given that increasing electron density in an aromatic ring makes it... | Course Hero](https://1.bp.blogspot.com/-I_v61ogLSBQ/VMzKnrqtx6I/AAAAAAAALBA/MtPkFiv3Xac/s1600/rescedon.jpg) Solved] Given that increasing electron density in an aromatic ring makes it… | Course Hero – #26
Solved] Given that increasing electron density in an aromatic ring makes it… | Course Hero – #26
 Diverse strategies for transition metal catalyzed distal C(sp 3 )–H functionalizations – Chemical Science (RSC Publishing) DOI:10.1039/D0SC04676K – #27
Diverse strategies for transition metal catalyzed distal C(sp 3 )–H functionalizations – Chemical Science (RSC Publishing) DOI:10.1039/D0SC04676K – #27
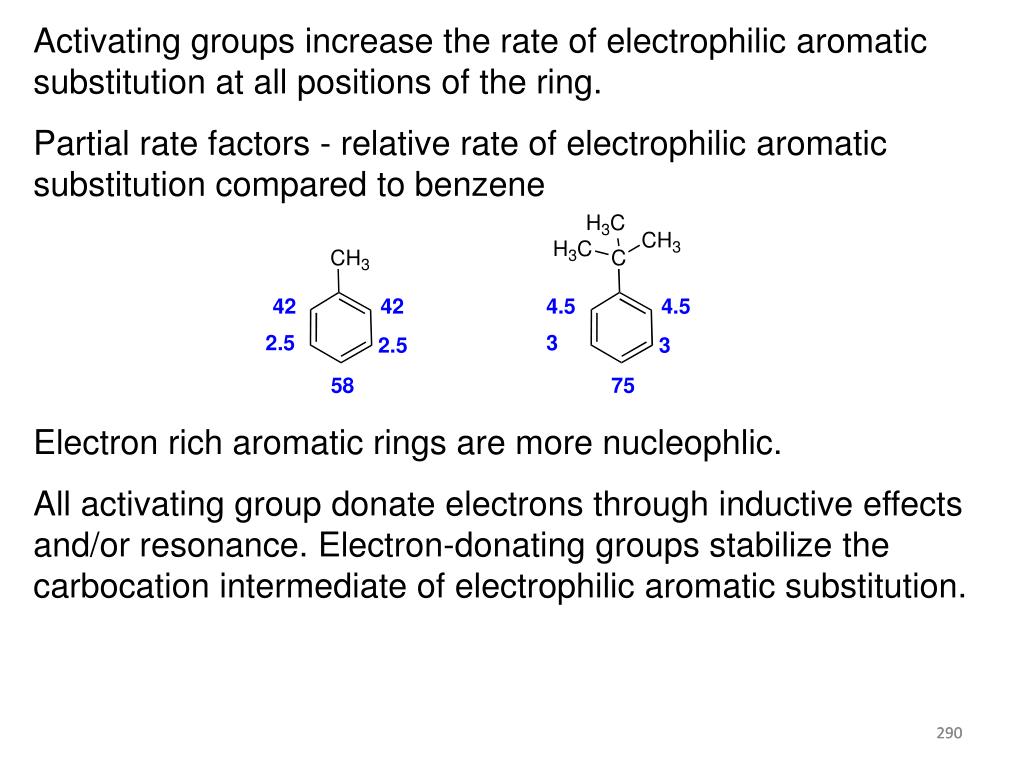 Frontiers | Reductive Functionalization of Amides in Synthesis and for Modification of Bioactive Compounds – #28
Frontiers | Reductive Functionalization of Amides in Synthesis and for Modification of Bioactive Compounds – #28
 Organocatalytic asymmetric arylation of indoles enabled by azo groups | Nature Chemistry – #29
Organocatalytic asymmetric arylation of indoles enabled by azo groups | Nature Chemistry – #29
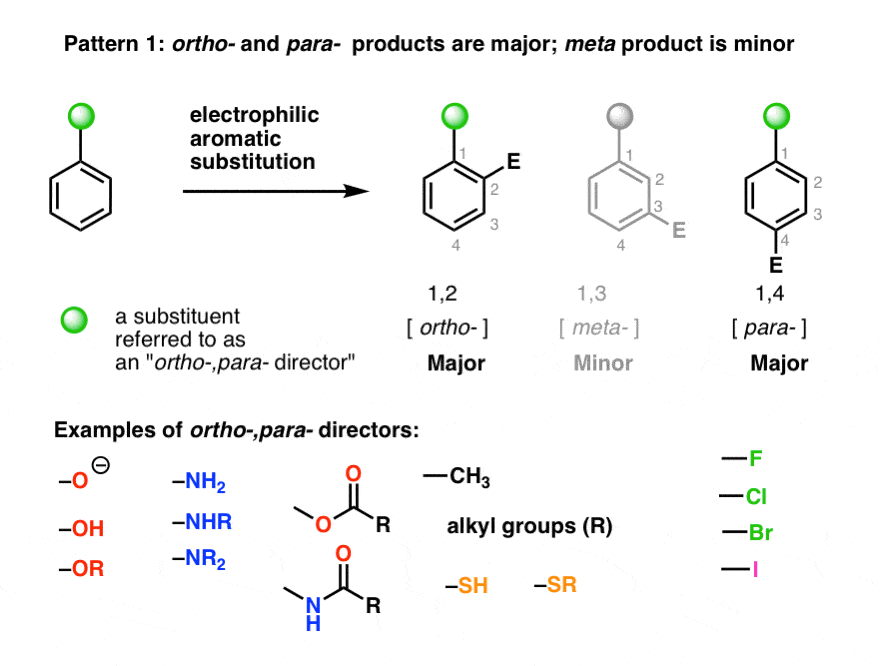 CONJUGATES COMPRISING HYDROXYALKYL STARCH AND A CYTOTOXIC AGENT AND PROCESS FOR THEIR PREPARATION – diagram, schematic, and image 81 – #30
CONJUGATES COMPRISING HYDROXYALKYL STARCH AND A CYTOTOXIC AGENT AND PROCESS FOR THEIR PREPARATION – diagram, schematic, and image 81 – #30
 Chapter 11 Arenes and Aromaticity – #31
Chapter 11 Arenes and Aromaticity – #31
 Chemistry of Benzene: Electrophilic Aromatic Substitution – ppt download – #32
Chemistry of Benzene: Electrophilic Aromatic Substitution – ppt download – #32
 Coatings | Free Full-Text | Current Progress on the Surface Chemical Modification of Carbonaceous Materials – #33
Coatings | Free Full-Text | Current Progress on the Surface Chemical Modification of Carbonaceous Materials – #33
 SOLVED: Explain why activating groups are ortho para-directing in an electrophilic aromatic substitution reaction? Explain why most deactivating groups are meta-directing in an electrophilic aromatic substitution reaction. Draw the major product, including – #34
SOLVED: Explain why activating groups are ortho para-directing in an electrophilic aromatic substitution reaction? Explain why most deactivating groups are meta-directing in an electrophilic aromatic substitution reaction. Draw the major product, including – #34
 SOLVED: Draw the major organic product(s) for the reaction. Multiple products may be drawn in one box, in any order. Charges and nonbonding electrons do not need to be included. Select Draw – #35
SOLVED: Draw the major organic product(s) for the reaction. Multiple products may be drawn in one box, in any order. Charges and nonbonding electrons do not need to be included. Select Draw – #35
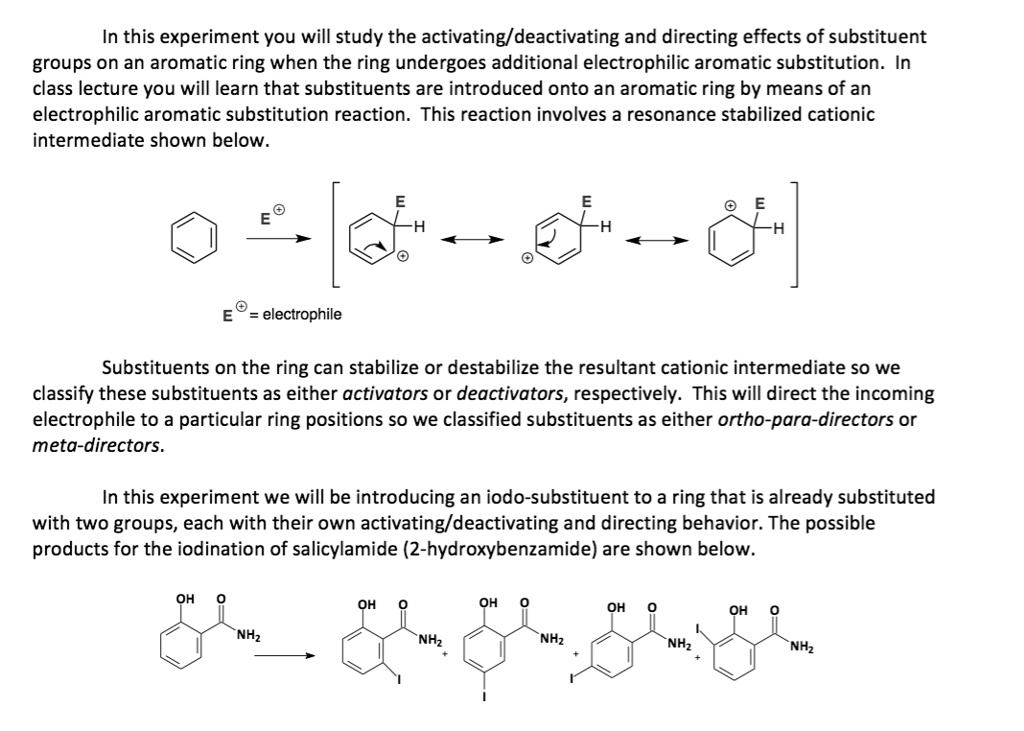
 SOLVED: In this experiment, you will study the activating/deactivating and directing effects of substituent groups on an aromatic ring when the ring undergoes additional electrophilic aromatic substitution. In the class lecture, you – #36
SOLVED: In this experiment, you will study the activating/deactivating and directing effects of substituent groups on an aromatic ring when the ring undergoes additional electrophilic aromatic substitution. In the class lecture, you – #36
- electron donating groups
- electron donating and withdrawing groups table
- ewg and edg chart
 Installing the “magic methyl” – C–H methylation in synthesis – Chemical Society Reviews (RSC Publishing) DOI:10.1039/D0CS00973C – #37
Installing the “magic methyl” – C–H methylation in synthesis – Chemical Society Reviews (RSC Publishing) DOI:10.1039/D0CS00973C – #37
 The Artery | CEPT – Portfolio – #38
The Artery | CEPT – Portfolio – #38
 SOLUTION: Nucleophilic Substitution at Carbonyl Groups Paper – Studypool – #39
SOLUTION: Nucleophilic Substitution at Carbonyl Groups Paper – Studypool – #39
 The organic compounds that give the following qualitative analysis is:TestInference(a) Dil. $\\text{ HCl }$Insoluble(b) $\\text{ NaOH }$solutionsoluble(c) $\\text{ B}{{\\text{r}}_{\\text{2}}}\\text{ \/ water }$ Decolourisation(A)\n \n \n \n \n (B)\n \n … – #40
The organic compounds that give the following qualitative analysis is:TestInference(a) Dil. $\\text{ HCl }$Insoluble(b) $\\text{ NaOH }$solutionsoluble(c) $\\text{ B}{{\\text{r}}_{\\text{2}}}\\text{ \/ water }$ Decolourisation(A)\n \n \n \n \n (B)\n \n … – #40
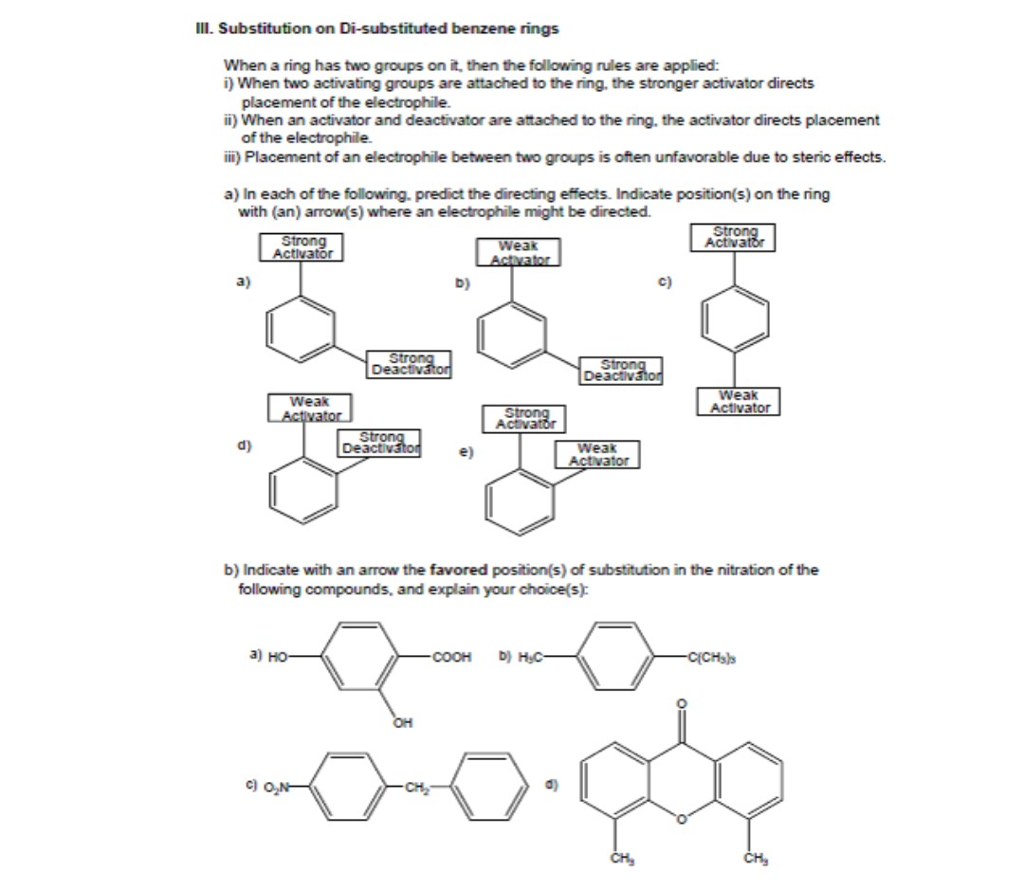
 Solved Substitution on Di-substituted benzene rings When a | Chegg.com – #41
Solved Substitution on Di-substituted benzene rings When a | Chegg.com – #41
 Solved 1. Which electrophiles deactivate a benzene ring? | Chegg.com – #42
Solved 1. Which electrophiles deactivate a benzene ring? | Chegg.com – #42
 How do activating and deactivating groups affect the reactivity of a benzene ring? – Quora – #43
How do activating and deactivating groups affect the reactivity of a benzene ring? – Quora – #43
 Explain sni and snar substitution reaction ? – #44
Explain sni and snar substitution reaction ? – #44
 Organic Chemistry Lecture Notes (Chapter 17) – Electrophilic Aromatic Substitution – Although – Studocu – #45
Organic Chemistry Lecture Notes (Chapter 17) – Electrophilic Aromatic Substitution – Although – Studocu – #45
 DIRECTIVE INFLUENCE OF ELECTRON WITHDRAWING GROUPS AND ELECTRON DONATING GROUPS ON BENZENE RING – YouTube – #46
DIRECTIVE INFLUENCE OF ELECTRON WITHDRAWING GROUPS AND ELECTRON DONATING GROUPS ON BENZENE RING – YouTube – #46
 Experience bliss at a new moon meditation – St George News – #47
Experience bliss at a new moon meditation – St George News – #47
 Pharmacologic ATF6 activating compounds are metabolically activated to selectively modify endoplasmic reticulum proteins | eLife – #48
Pharmacologic ATF6 activating compounds are metabolically activated to selectively modify endoplasmic reticulum proteins | eLife – #48
 Chapter 16-Benzene-Electrophilic Aromatic Substitution – Chapter 16: Benzene: Electrophilic Aromatic – Studocu – #49
Chapter 16-Benzene-Electrophilic Aromatic Substitution – Chapter 16: Benzene: Electrophilic Aromatic – Studocu – #49
 Electrophilic aromatic substitution – Wikipedia – #50
Electrophilic aromatic substitution – Wikipedia – #50
 Solved Toluene Anisele Although both methyl and methoxy are | Chegg.com – #51
Solved Toluene Anisele Although both methyl and methoxy are | Chegg.com – #51
 Explain why the trifluoromethyl (CF_3) group is metadirecting in electrophilic aromatic substitution. Would you expect CF_3 to be activating or deactivating?Why? | Homework.Study.com – #52
Explain why the trifluoromethyl (CF_3) group is metadirecting in electrophilic aromatic substitution. Would you expect CF_3 to be activating or deactivating?Why? | Homework.Study.com – #52
 Solved When a ring has two groups on it then toe following | Chegg.com – #53
Solved When a ring has two groups on it then toe following | Chegg.com – #53
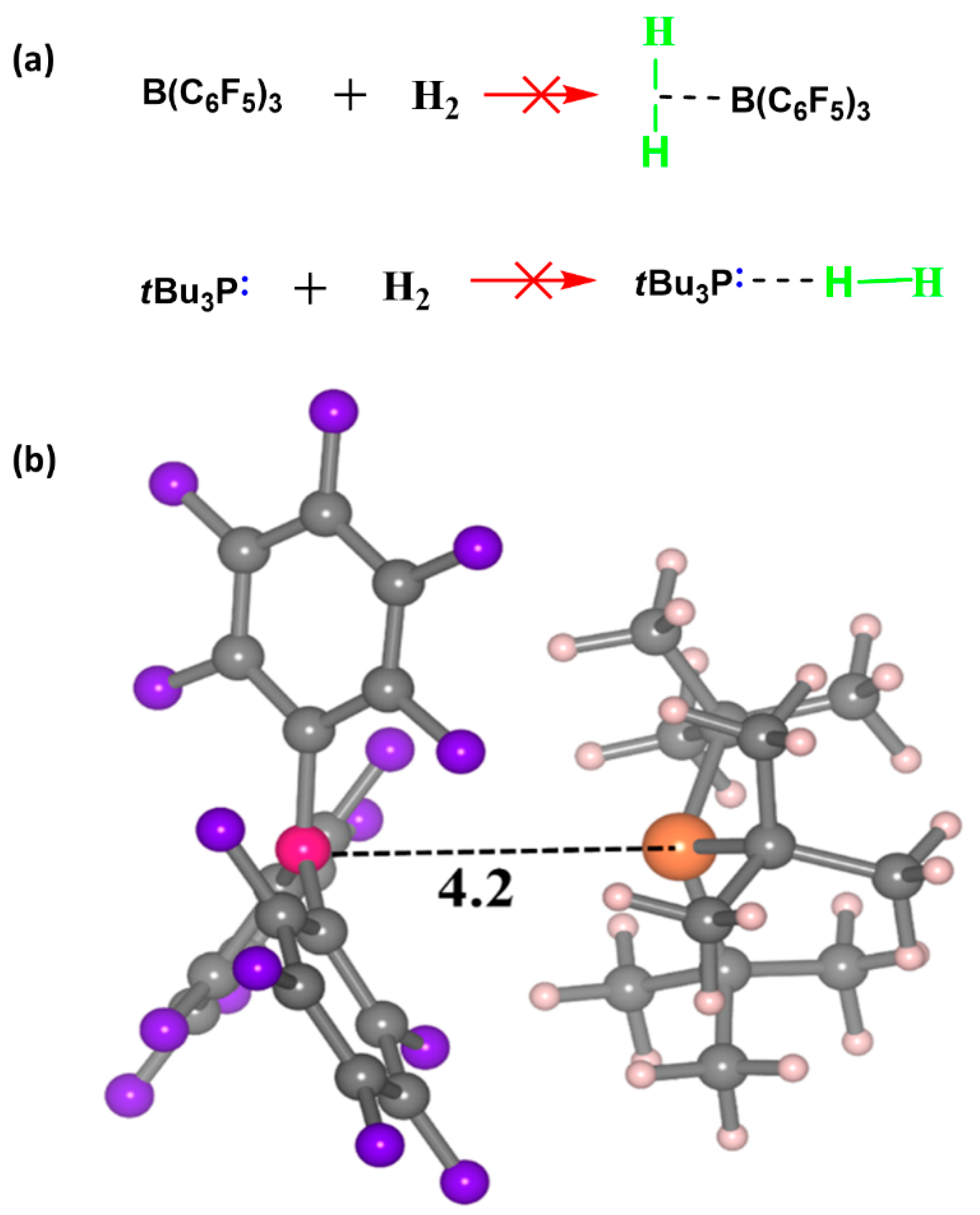 ACS Spring 2024 – Current & Upcoming Schedule – American Chemical Society – #54
ACS Spring 2024 – Current & Upcoming Schedule – American Chemical Society – #54
 March 08 1 Dr. Abdullah Saleh – #55
March 08 1 Dr. Abdullah Saleh – #55
 AP PGCET POLYMER SCIENCE Syllabus – IndCareer Docs – #56
AP PGCET POLYMER SCIENCE Syllabus – IndCareer Docs – #56
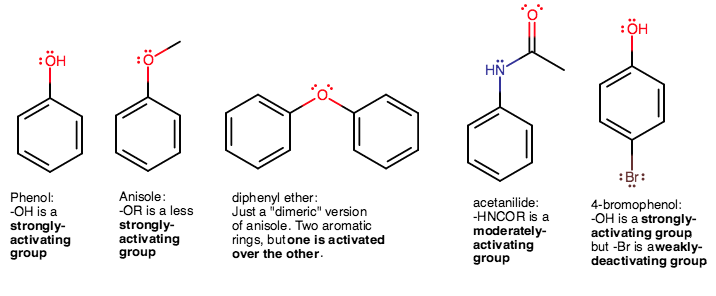 Nitration of Benzene – Chemistry Steps – #57
Nitration of Benzene – Chemistry Steps – #57
JoVE Science Education > Reactions of Aromatic Compounds – #58
![Solved] Electrophilic Aromatic Bromination and Organic Synthesis... | Course Hero Solved] Electrophilic Aromatic Bromination and Organic Synthesis... | Course Hero](https://static.doubtnut.com/ss/web-overlay-thumb/2816955.webp) Solved] Electrophilic Aromatic Bromination and Organic Synthesis… | Course Hero – #59
Solved] Electrophilic Aromatic Bromination and Organic Synthesis… | Course Hero – #59
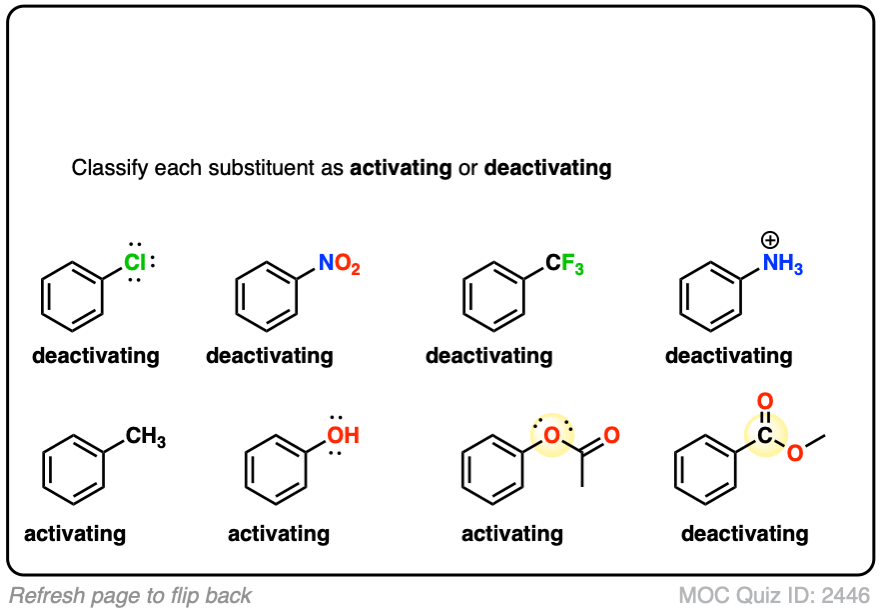 In silico rationalisation of selectivity and reactivity in Pd-catalysed C–H activation reactions – #60
In silico rationalisation of selectivity and reactivity in Pd-catalysed C–H activation reactions – #60
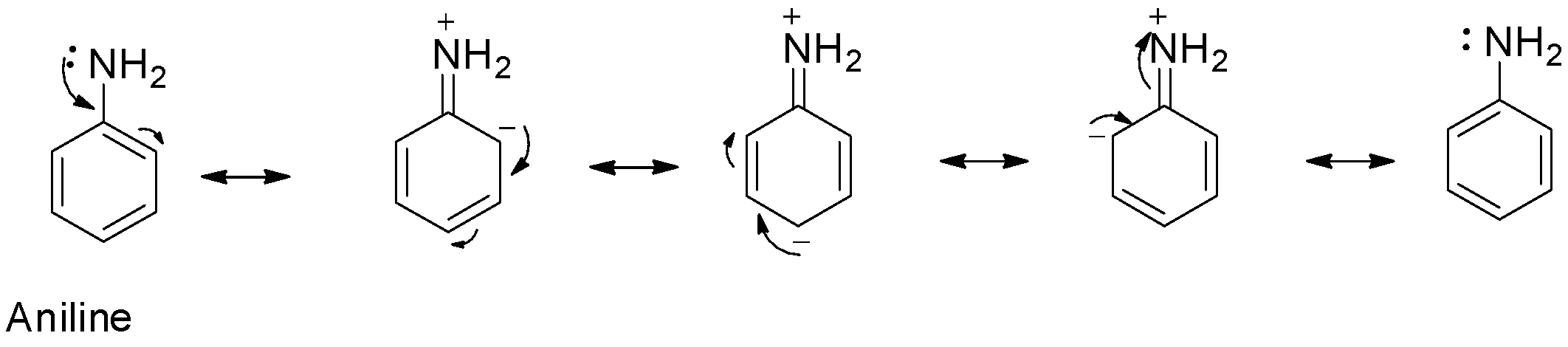
 ng Uy resonance. Therefore, activating ellect of -NHCOCH, group is than that of amino group (b) Nitration: Direct nitration of aniline yields tarry products in addition to the nitro derivatives. Moreover, on – #61
ng Uy resonance. Therefore, activating ellect of -NHCOCH, group is than that of amino group (b) Nitration: Direct nitration of aniline yields tarry products in addition to the nitro derivatives. Moreover, on – #61
 Explain what are the steps in the mechanism, for nitration of methyl benzoate, and the favored/stable positions in the benzene ring. Provide a brief example to your explination. | Homework.Study.com – #62
Explain what are the steps in the mechanism, for nitration of methyl benzoate, and the favored/stable positions in the benzene ring. Provide a brief example to your explination. | Homework.Study.com – #62
 SOLUTION: 5 1 rrt arsn – Studypool – #63
SOLUTION: 5 1 rrt arsn – Studypool – #63
 Experiment 7 Handout 2023 – EXPERIMENT 5: ELECTROPHILIC AROMATIC SUBSTITUTION OF SALICYLAMIDE – Studocu – #64
Experiment 7 Handout 2023 – EXPERIMENT 5: ELECTROPHILIC AROMATIC SUBSTITUTION OF SALICYLAMIDE – Studocu – #64
 Effects of Substituent On Benzene Ring | PDF | Physical Chemistry | Organic Chemistry – #65
Effects of Substituent On Benzene Ring | PDF | Physical Chemistry | Organic Chemistry – #65
 Peptides | SpringerLink – #66
Peptides | SpringerLink – #66
 NEET Personal Mentorship by PCB Point Seniors on Instagram: “Resonance 📝 Credit – @_dropper_to_topper_ Your one stop point for all the FLASHCARDS, MNEMONICS, NOTES, TESTS, VIDEO LECTURES = “The PCB Point” mobile – #67
NEET Personal Mentorship by PCB Point Seniors on Instagram: “Resonance 📝 Credit – @_dropper_to_topper_ Your one stop point for all the FLASHCARDS, MNEMONICS, NOTES, TESTS, VIDEO LECTURES = “The PCB Point” mobile – #67
 Suggest a synthetic route from benzene to 1,3-dibromo-2-nitr | Quizlet – #68
Suggest a synthetic route from benzene to 1,3-dibromo-2-nitr | Quizlet – #68
![Solved -NH, THR, NR ] Increasing activation ÖR activating | Chegg.com Solved -NH, THR, NR ] Increasing activation ÖR activating | Chegg.com](https://cdn1.byjus.com/wp-content/uploads/2016/01/Directive-Influence-Of-Functional-Group-In-Mono-Substituted-Benzene-02-700x221.png) Solved -NH, THR, NR ] Increasing activation ÖR activating | Chegg.com – #69
Solved -NH, THR, NR ] Increasing activation ÖR activating | Chegg.com – #69
 F.A.Qs. – #70
F.A.Qs. – #70
 Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids | Nature Communications – #71
Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids | Nature Communications – #71
 How to Identify Activated Microglia | Proteintech Group – #72
How to Identify Activated Microglia | Proteintech Group – #72
 How To Find The Most Reactive Position In Electrophilic Aromatic Substitution Reactions? – #73
How To Find The Most Reactive Position In Electrophilic Aromatic Substitution Reactions? – #73
 SOLUTION: Orientation and reactivity in disubstituted benzene – Studypool – #74
SOLUTION: Orientation and reactivity in disubstituted benzene – Studypool – #74
 How Do You Know Which Group Is Activating And Deactivating? – #75
How Do You Know Which Group Is Activating And Deactivating? – #75
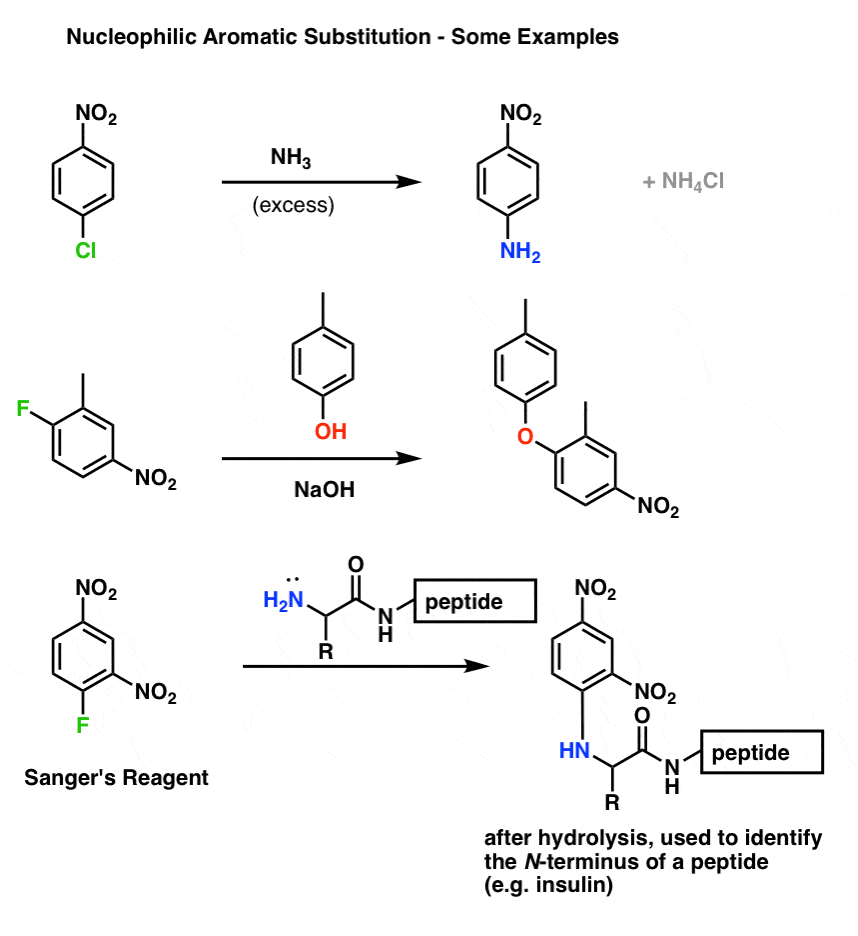
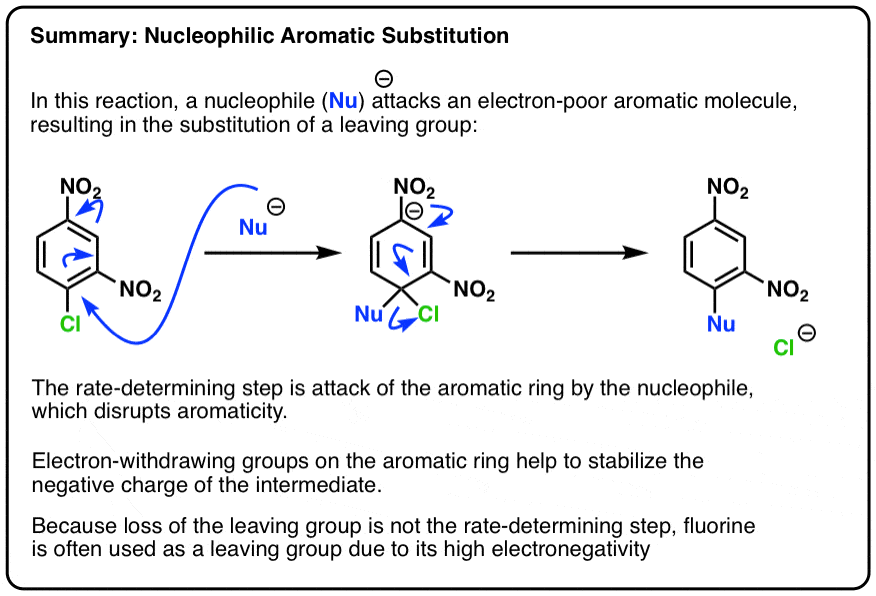
 Nucleophilic Aromatic Substitution: Introduction and Mechanism – #76
Nucleophilic Aromatic Substitution: Introduction and Mechanism – #76
 Activating and Deactivating Groups In Electrophilic Aromatic Substitution | Substitute, Organic chemistry, Aromatic – #77
Activating and Deactivating Groups In Electrophilic Aromatic Substitution | Substitute, Organic chemistry, Aromatic – #77
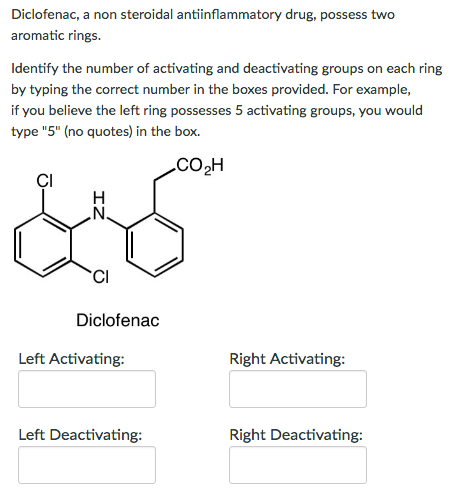 Solved 4. Having two or more substituents in a ring can work | Chegg.com – #78
Solved 4. Having two or more substituents in a ring can work | Chegg.com – #78
- meta directing groups resonance
- activating substituents on a benzene ring
- electron withdrawing groups list pdf
 LALBABA COLLEGE CHEMISTRY – GENERAL INTERNAL ASSESSMENT CC4/GE4 − 2021 FULL MARKS – 10 TIME – HALF HRS ANSWER ANY TEN QU – #79
LALBABA COLLEGE CHEMISTRY – GENERAL INTERNAL ASSESSMENT CC4/GE4 − 2021 FULL MARKS – 10 TIME – HALF HRS ANSWER ANY TEN QU – #79
 Exercise 115 – Directing-Activation.cdx – #80
Exercise 115 – Directing-Activation.cdx – #80
 Scheme 8: Competition between ring formation para-or meta-to an… | Download Scientific Diagram – #81
Scheme 8: Competition between ring formation para-or meta-to an… | Download Scientific Diagram – #81
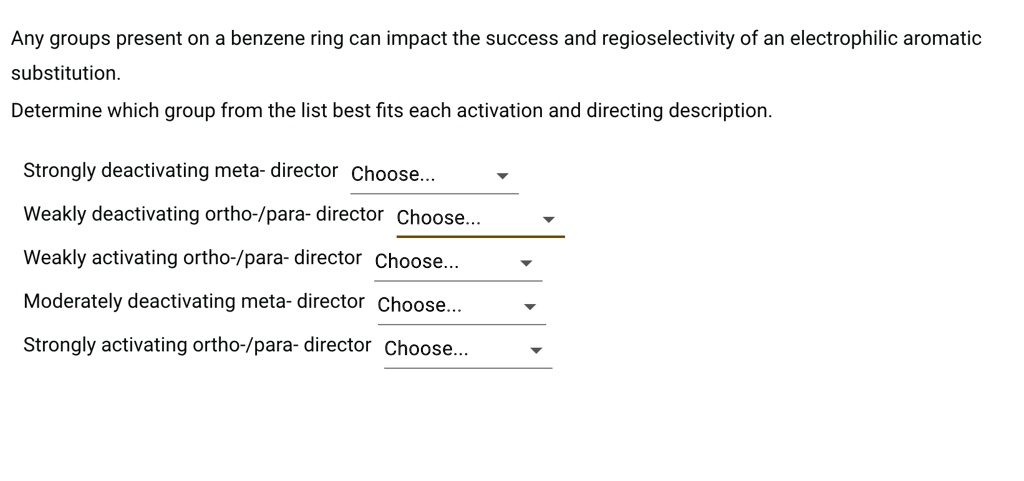
 SOLVED: Any groups present on a benzene ring can impact the success and regioselectivity of an electrophilic aromatic substitution Determine which group from the list best fits each activation and directing description. – #82
SOLVED: Any groups present on a benzene ring can impact the success and regioselectivity of an electrophilic aromatic substitution Determine which group from the list best fits each activation and directing description. – #82
 Accelerators for Amine Curing Agents – Polymer Innovation Blog – #83
Accelerators for Amine Curing Agents – Polymer Innovation Blog – #83
 SOLVED: Which statement is false about electron withdrawing releasing groups? All electron-donating groups are activating groups and all are ortho-para directors. With the exception of halogen substituents, all electron-withdrawing groups are deactivating – #84
SOLVED: Which statement is false about electron withdrawing releasing groups? All electron-donating groups are activating groups and all are ortho-para directors. With the exception of halogen substituents, all electron-withdrawing groups are deactivating – #84
Ph Ph Meo OK (III) (IV) Order of rate of electrophilic addition reaction with HBr will be (a) IV > II > I > III (b)I> II > III > IV (C) – #85
 Illustrated Glossary of Organic Chemistry – Phenyl group – #86
Illustrated Glossary of Organic Chemistry – Phenyl group – #86
 Activating and deactivating group | PPT – #87
Activating and deactivating group | PPT – #87
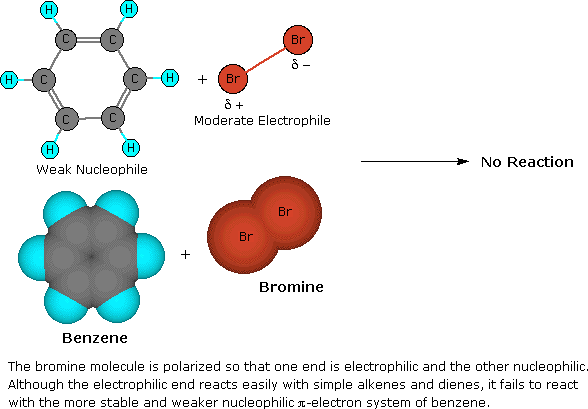
 Wouldn’t No reaction occur? : r/OrganicChemistry – #88
Wouldn’t No reaction occur? : r/OrganicChemistry – #88
- ortho para meta activating deactivating
- meta directing groups
- is och3 activating or deactivating
- activator and deactivator in benzene ring
- how to identify electron withdrawing groups
- meta directing mechanism
 Synthesis of polynitro compounds. Hexasubstituted benzenes | The Journal of Organic Chemistry – #89
Synthesis of polynitro compounds. Hexasubstituted benzenes | The Journal of Organic Chemistry – #89
 Chapter 16: Reactions of Aromatic Compounds Flashcards | Quizlet – #90
Chapter 16: Reactions of Aromatic Compounds Flashcards | Quizlet – #90
 General reaction of pyrimidine ring 23 and 29-32, singly activated… | Download Scientific Diagram – #91
General reaction of pyrimidine ring 23 and 29-32, singly activated… | Download Scientific Diagram – #91
 Organic π-Systems | Chemogenesis – #92
Organic π-Systems | Chemogenesis – #92
 Electrophilic substitutions of mono-substituted aromatic rings – #93
Electrophilic substitutions of mono-substituted aromatic rings – #93
 Why is the OH group more activating than OR despite more +I effect of R group? – Quora – #94
Why is the OH group more activating than OR despite more +I effect of R group? – Quora – #94
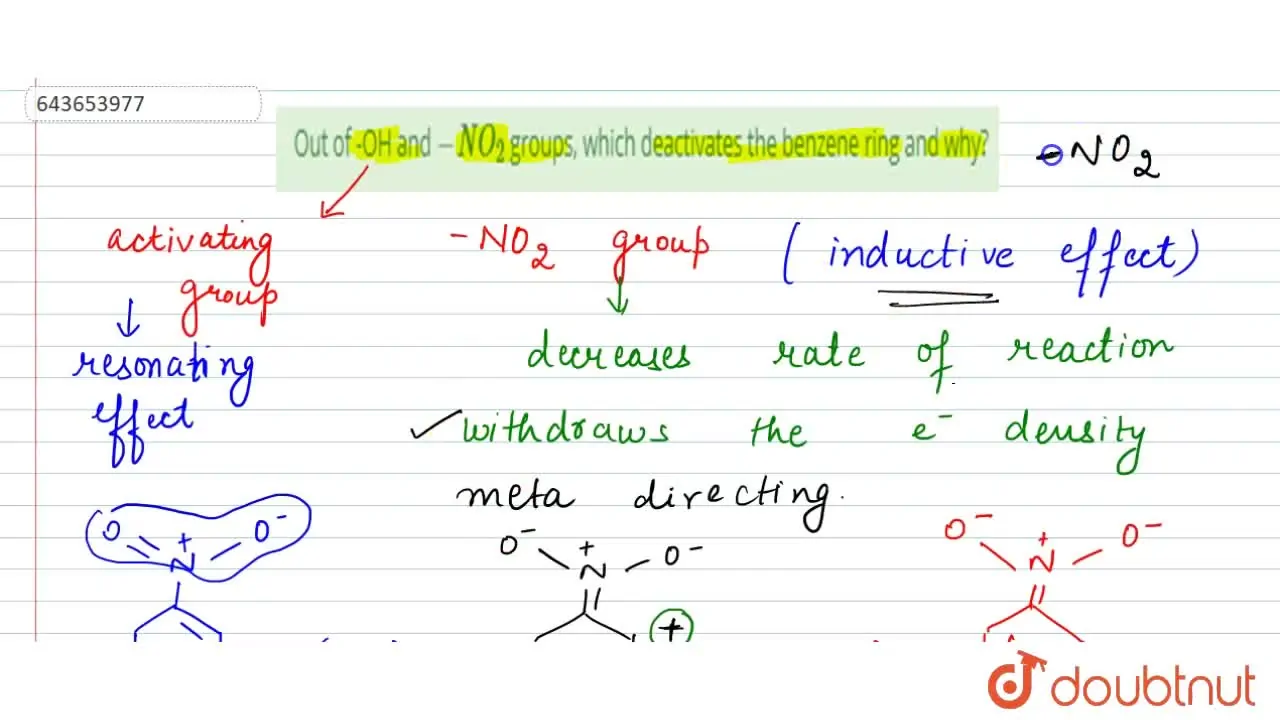
 Why does the NO2 group show its effect only in ortho? – #95
Why does the NO2 group show its effect only in ortho? – #95
 Why is chlorine ortho- and para-directing but deactivating? What is the reaction, and state the effect associated with it? – Quora – #96
Why is chlorine ortho- and para-directing but deactivating? What is the reaction, and state the effect associated with it? – Quora – #96
 Substituent effects and local molecular shape correlations – Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C3CP55192J – #97
Substituent effects and local molecular shape correlations – Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C3CP55192J – #97
 Decoding Directing Groups and Their Pivotal Role in C−H Activation – Murali – 2021 – Chemistry – A European Journal – Wiley Online Library – #98
Decoding Directing Groups and Their Pivotal Role in C−H Activation – Murali – 2021 – Chemistry – A European Journal – Wiley Online Library – #98
 Directing Groups–Activating and Deactivating Benzene (via Resonance and Induction) – YouTube – #99
Directing Groups–Activating and Deactivating Benzene (via Resonance and Induction) – YouTube – #99
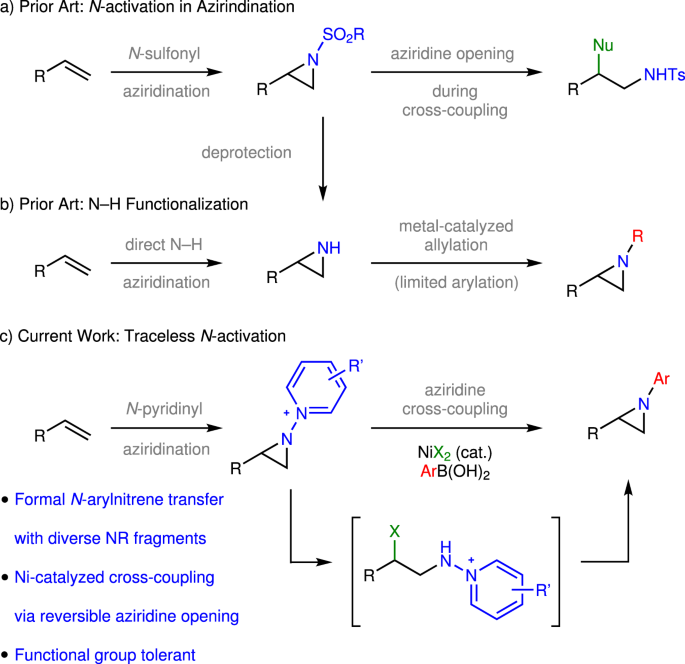
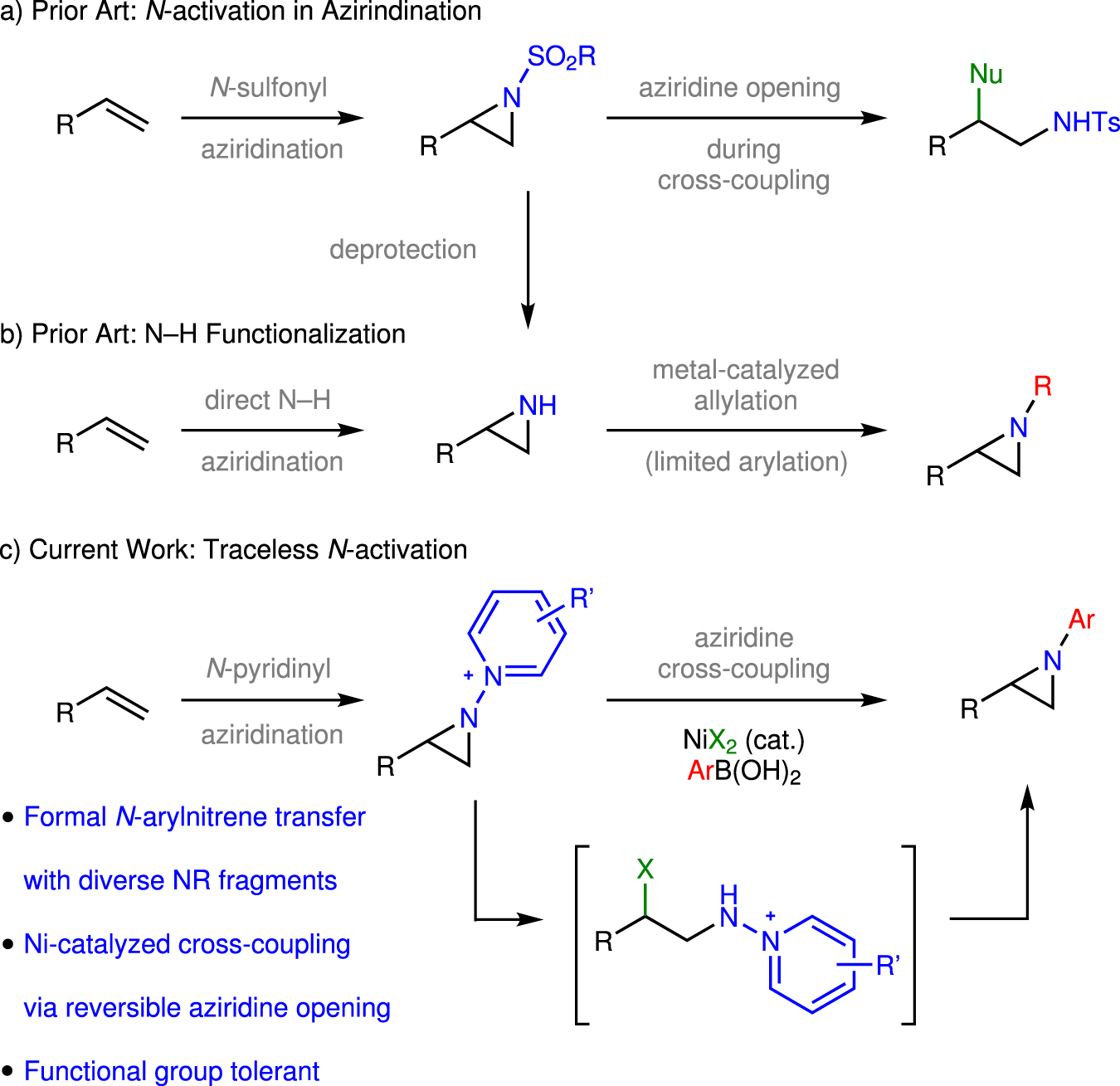
 Catalysts | Free Full-Text | Traceless Directing Groups in Sustainable Metal-Catalyzed C–H Activation – #100
Catalysts | Free Full-Text | Traceless Directing Groups in Sustainable Metal-Catalyzed C–H Activation – #100
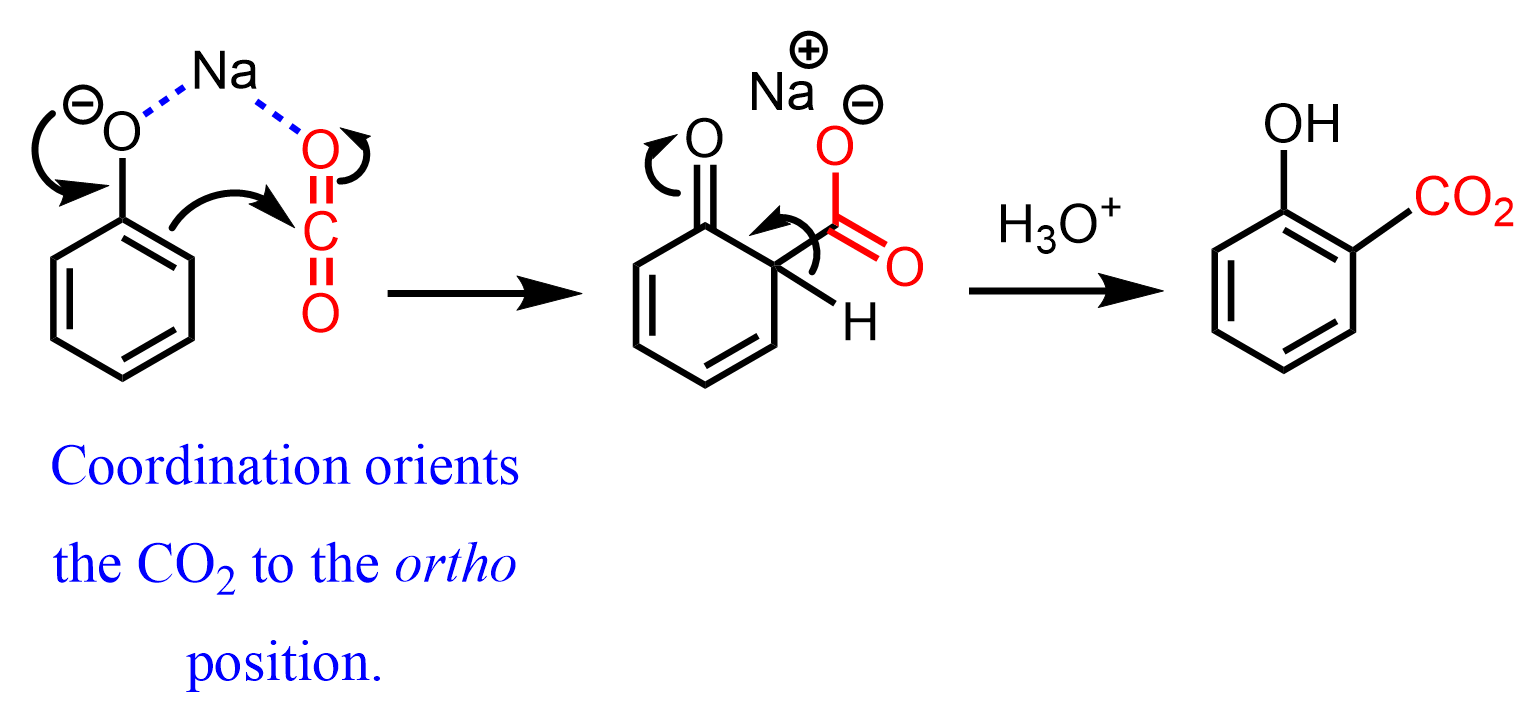 Untitled – #101
Untitled – #101
 SOLVED: Compare the following two benzene rings and indicate which is slower and which is faster in EAS, providing the main fundamental reason: Ring A reacts slower because it is connected to – #102
SOLVED: Compare the following two benzene rings and indicate which is slower and which is faster in EAS, providing the main fundamental reason: Ring A reacts slower because it is connected to – #102
 Furan undergoes electrophilic aromatic substitution more readily … | Channels for Pearson+ – #103
Furan undergoes electrophilic aromatic substitution more readily … | Channels for Pearson+ – #103
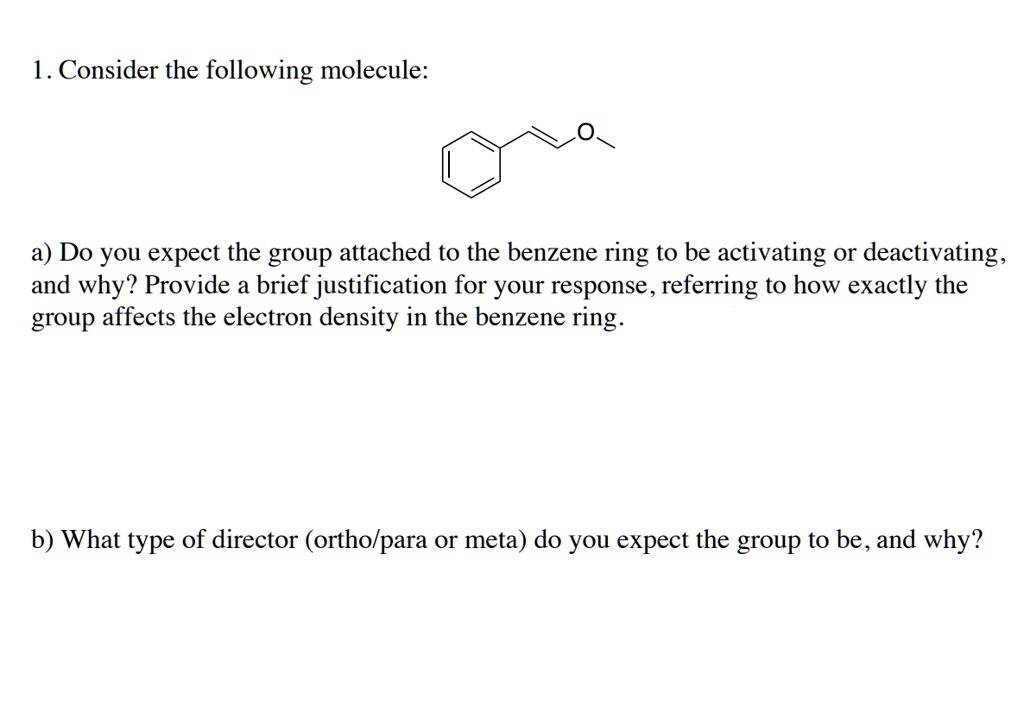
 SOLVED: 1) Please explain electrophilic aromatic substitution: Is the benzene ring behaving like a nucleophile or an electrophile in these reactions? 2) Please explain the directing effects of an activating group that – #104
SOLVED: 1) Please explain electrophilic aromatic substitution: Is the benzene ring behaving like a nucleophile or an electrophile in these reactions? 2) Please explain the directing effects of an activating group that – #104
- ortho para directing activating and deactivating groups
- activating and deactivating groups
- electron withdrawing groups chart
![Solved] Please answer thanks for me | Course Hero Solved] Please answer thanks for me | Course Hero](https://media.cheggcdn.com/study/150/150aab7b-d363-4a5b-9270-e37e029f97b1/image.png) Solved] Please answer thanks for me | Course Hero – #105
Solved] Please answer thanks for me | Course Hero – #105
 Summary model for the role of the chloride ring in the assembly of… | Download Scientific Diagram – #106
Summary model for the role of the chloride ring in the assembly of… | Download Scientific Diagram – #106
 School of Chemistry | Facebook – #107
School of Chemistry | Facebook – #107
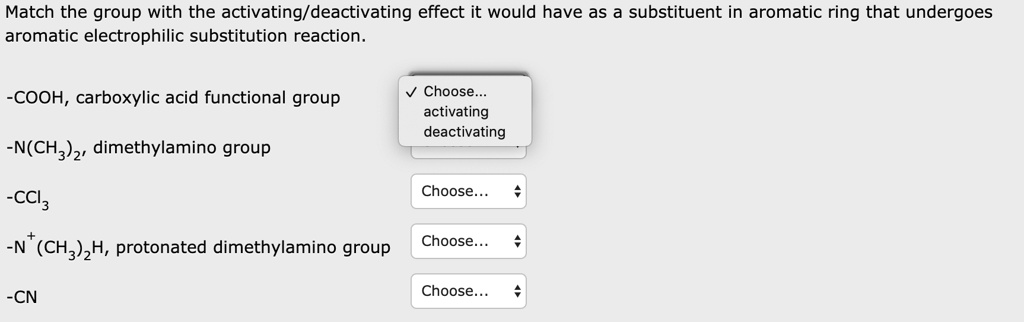
 SOLVED: Match the group with the activating/deactivating effect it would have as a substituent in an aromatic ring that undergoes aromatic electrophilic substitution reaction. COOH, carboxylic acid functional group Choose: activating N(CH3)2, – #108
SOLVED: Match the group with the activating/deactivating effect it would have as a substituent in an aromatic ring that undergoes aromatic electrophilic substitution reaction. COOH, carboxylic acid functional group Choose: activating N(CH3)2, – #108
 Toluene, when treated with Br2/FeCl3 gives p bromotoluene as the major product because the CH3 group: – #109
Toluene, when treated with Br2/FeCl3 gives p bromotoluene as the major product because the CH3 group: – #109
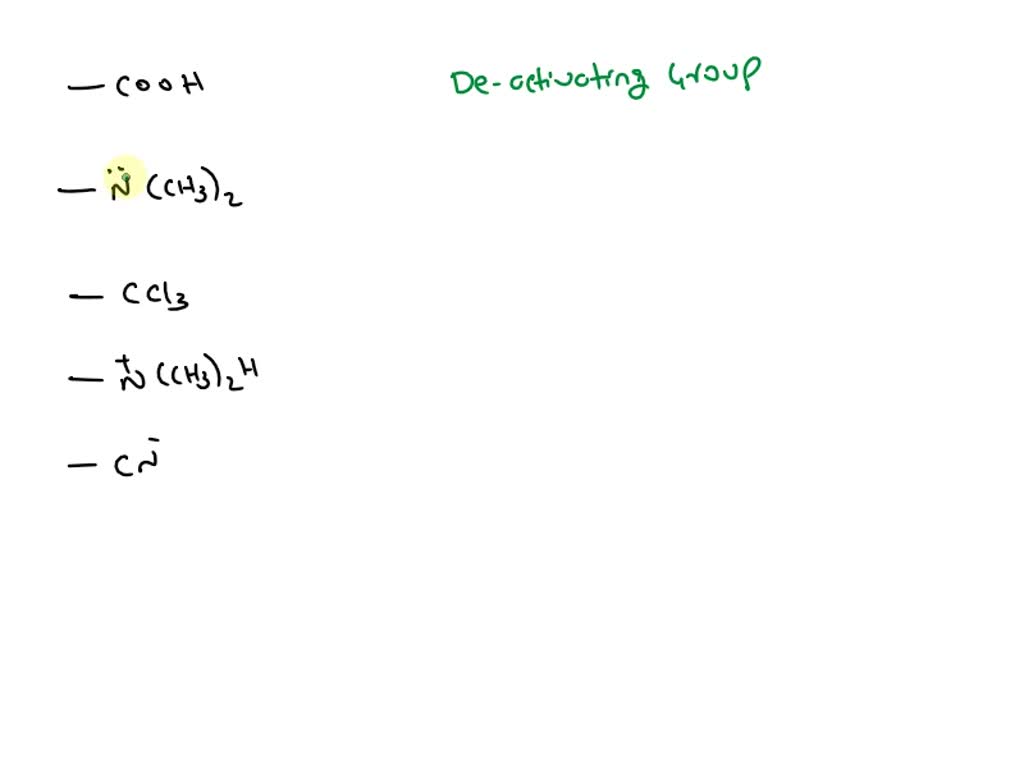 An analysis of electrophilic aromatic substitution: a “complex approach” – Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D0CP05245K – #110
An analysis of electrophilic aromatic substitution: a “complex approach” – Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D0CP05245K – #110
 Solved: Chapter 23 Problem 62P Solution | Organic Chemistry 0th Edition | Chegg.com – #111
Solved: Chapter 23 Problem 62P Solution | Organic Chemistry 0th Edition | Chegg.com – #111
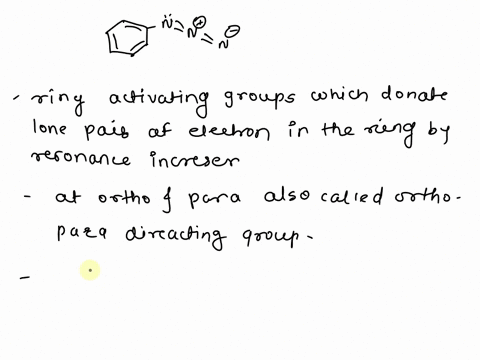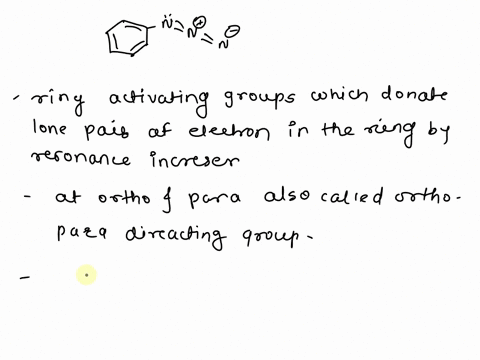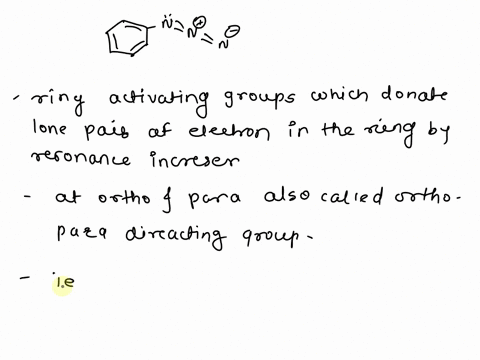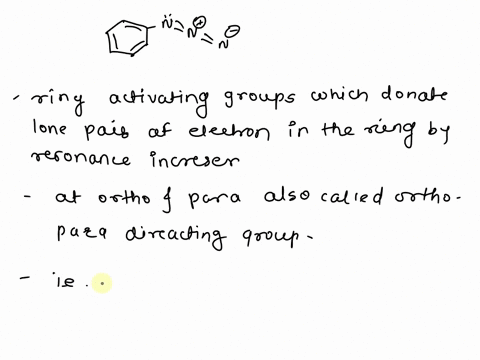 What is the difference between an activating group and a deactivating group in a benzene ring? – Quora – #112
What is the difference between an activating group and a deactivating group in a benzene ring? – Quora – #112
 Draw all resonance structures of nitrobenzene and explain why the nitro group (NO2) is meta-directing and deactivating on the benzene ring. | Homework.Study.com – #113
Draw all resonance structures of nitrobenzene and explain why the nitro group (NO2) is meta-directing and deactivating on the benzene ring. | Homework.Study.com – #113
- electron withdrawing groups
- inductive effect electron withdrawing groups
- activators vs deactivators
 PDF) Unlocking mild-condition benzene ring contraction using nonheme diiron N -oxygenase – #114
PDF) Unlocking mild-condition benzene ring contraction using nonheme diiron N -oxygenase – #114
 Aromatic Reactivity – #115
Aromatic Reactivity – #115
- electron withdrawing groups examples
- ring activating groups examples
- nucleophilic aromatic substitution
 Solved 2. Create aromatic rings with activating and | Chegg.com – #116
Solved 2. Create aromatic rings with activating and | Chegg.com – #116
 Structure of benzene – #117
Structure of benzene – #117
 SOLVED: Having two or more substituents in a ring can work for or against each other. For example, the following molecule has two activating groups and one deactivating group: Suppose a nitration – #118
SOLVED: Having two or more substituents in a ring can work for or against each other. For example, the following molecule has two activating groups and one deactivating group: Suppose a nitration – #118
 SOLUTION: Aromatic nucleophilic substitution reaction – Studypool – #119
SOLUTION: Aromatic nucleophilic substitution reaction – Studypool – #119
 9. I Il but nucleophilic than II (d) I is basic but nucleophilic than II 5. In chlorobenzene, the-Cl group: (a) Activates the benzene ring via resonance effect than des effect lan – #120
9. I Il but nucleophilic than II (d) I is basic but nucleophilic than II 5. In chlorobenzene, the-Cl group: (a) Activates the benzene ring via resonance effect than des effect lan – #120
 Aromatic Electrophilic Substitution – #121
Aromatic Electrophilic Substitution – #121
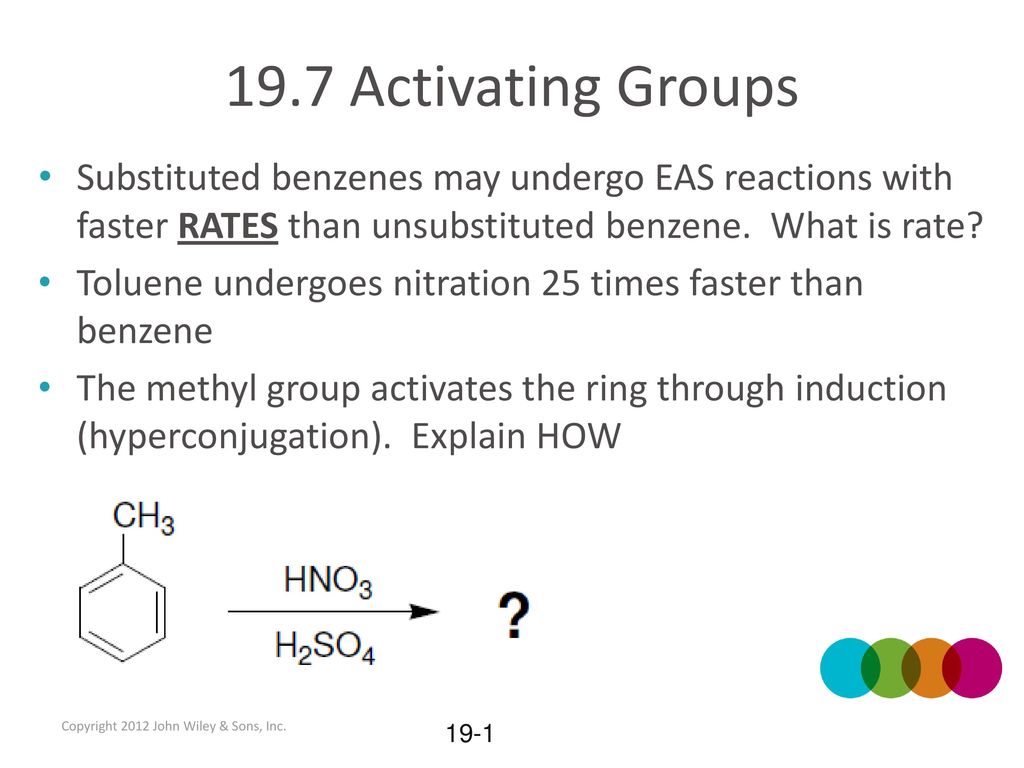
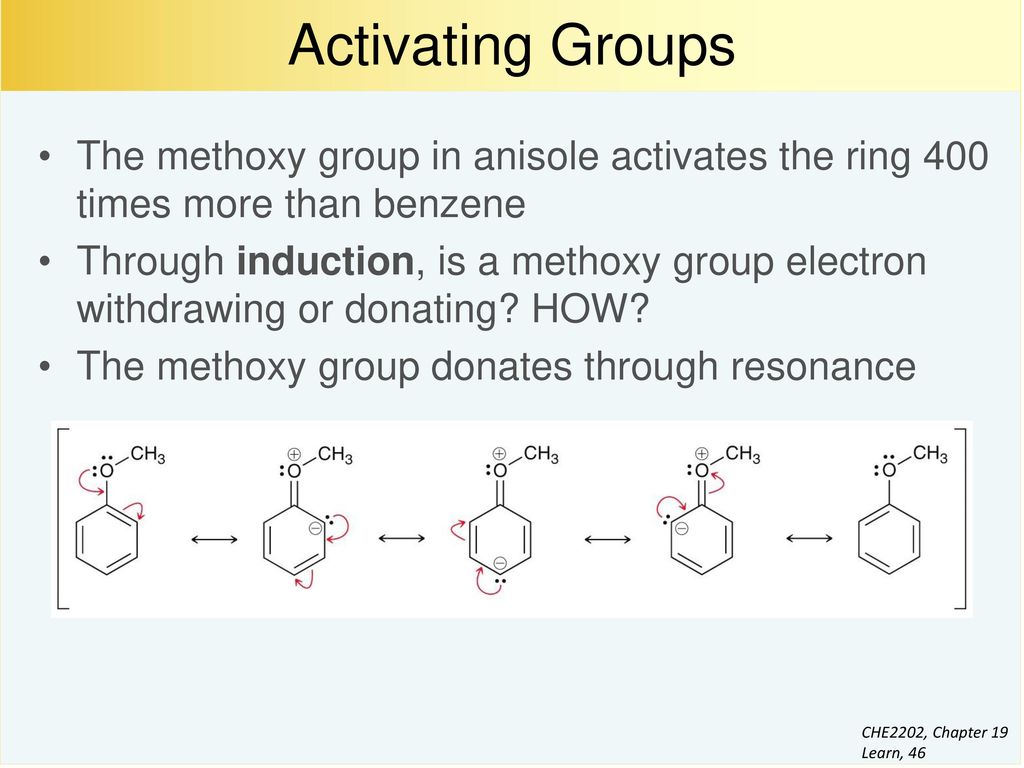
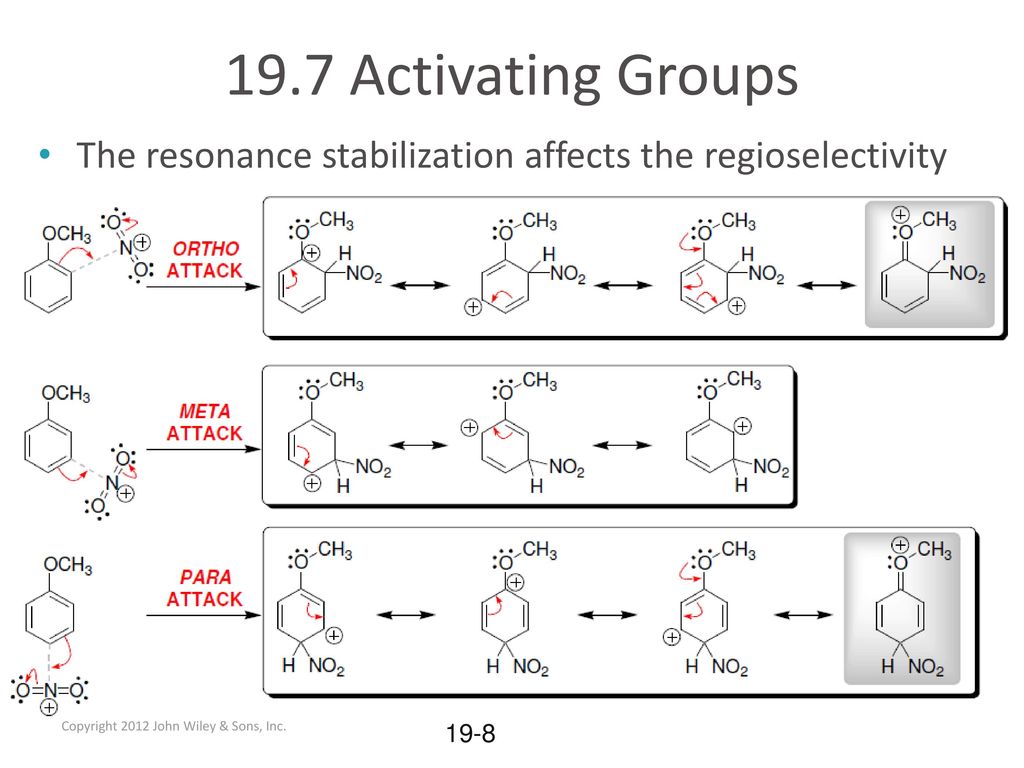
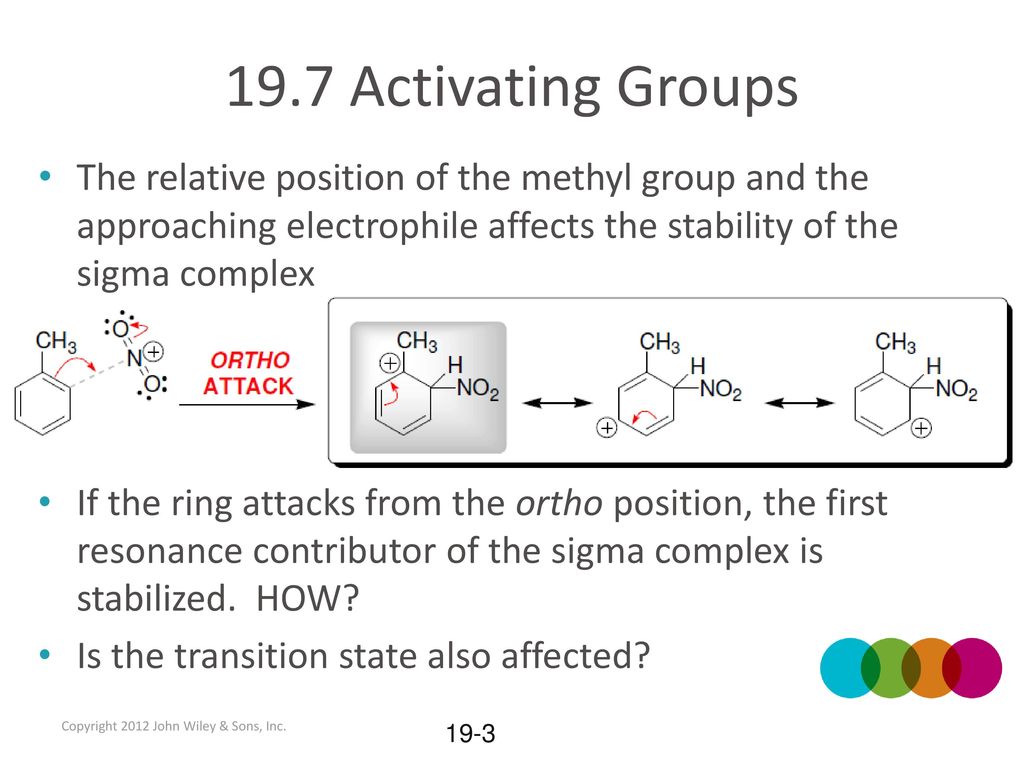
 19.7 Activating Groups Substituted benzenes may undergo EAS reactions with faster RATES than unsubstituted benzene. What is rate? Toluene undergoes nitration. – ppt download – #122
19.7 Activating Groups Substituted benzenes may undergo EAS reactions with faster RATES than unsubstituted benzene. What is rate? Toluene undergoes nitration. – ppt download – #122
 BenZene Ractions Dr Md Ashraful Alam Assistant Professor Department of Pharmaceutical Sciences. – ppt download – #123
BenZene Ractions Dr Md Ashraful Alam Assistant Professor Department of Pharmaceutical Sciences. – ppt download – #123
 NCERT Section – #124
NCERT Section – #124
 PPT – Electrophiles / Nucleophiles PowerPoint Presentation, free download – ID:4547422 – #125
PPT – Electrophiles / Nucleophiles PowerPoint Presentation, free download – ID:4547422 – #125
 −NO2 group is meta directing. Hence, (B) is correct. EXERCISE -2 1. Pyri.. – #126
−NO2 group is meta directing. Hence, (B) is correct. EXERCISE -2 1. Pyri.. – #126
 11: Why does acetylation of – NH2 group of aniline reduce its activity e.. – #127
11: Why does acetylation of – NH2 group of aniline reduce its activity e.. – #127
 Solved In this experiment you will study the | Chegg.com – #128
Solved In this experiment you will study the | Chegg.com – #128
 Chemical glycosylation – Wikipedia – #129
Chemical glycosylation – Wikipedia – #129
 Frontiers | Activation of the PPARγ Prevents Ferroptosis-Induced Neuronal Loss in Response to Intracerebral Hemorrhage Through Synergistic Actions With the Nrf2 – #130
Frontiers | Activation of the PPARγ Prevents Ferroptosis-Induced Neuronal Loss in Response to Intracerebral Hemorrhage Through Synergistic Actions With the Nrf2 – #130
 organic chemistry – activating and deactivating groups and directing effect – Chemistry Stack Exchange – #131
organic chemistry – activating and deactivating groups and directing effect – Chemistry Stack Exchange – #131
 17-rxns aromatic.pptx – #132
17-rxns aromatic.pptx – #132
 CH237 – Chapter 22 – Reactions of Benzene and it’s Derivatives Flashcards | Quizlet – #133
CH237 – Chapter 22 – Reactions of Benzene and it’s Derivatives Flashcards | Quizlet – #133
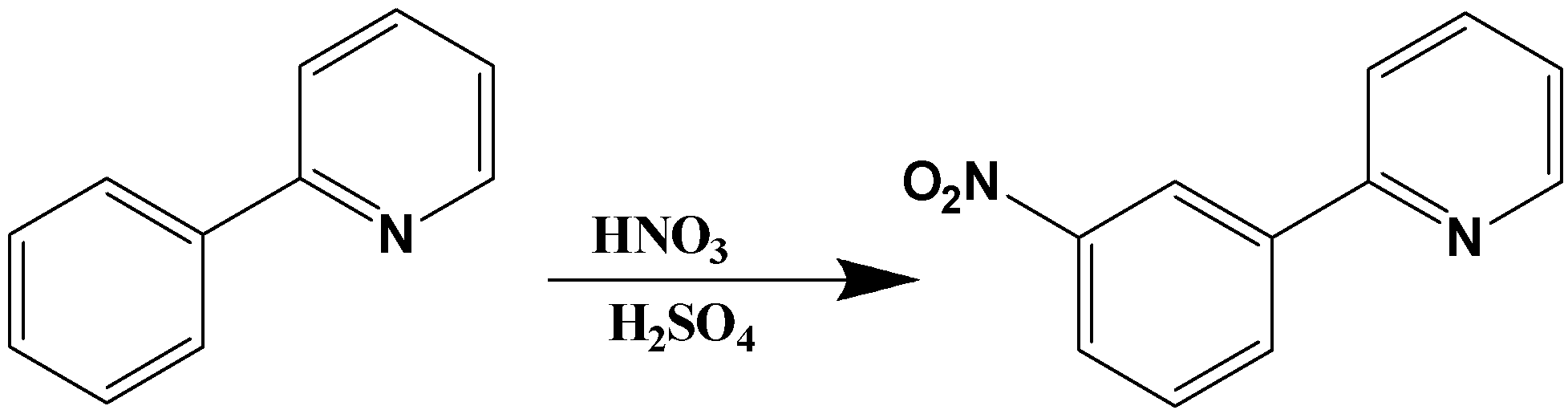 Ex 4 Azo Dyes – #134
Ex 4 Azo Dyes – #134
![Structure of Bis[tert-butyl (2-Lithiophenyl) Sulfide] [N,N,N',N'- Tetramethylethylenediaraine] Structure of Bis[tert-butyl (2-Lithiophenyl) Sulfide] [N,N,N',N'- Tetramethylethylenediaraine]](https://media.cheggcdn.com/media/538/5386b946-ffc6-4b1b-90c4-b25ffb2726b0/phpEEzAEJ.png) Structure of Bis[tert-butyl (2-Lithiophenyl) Sulfide] [N,N,N’,N’- Tetramethylethylenediaraine] – #135
Structure of Bis[tert-butyl (2-Lithiophenyl) Sulfide] [N,N,N’,N’- Tetramethylethylenediaraine] – #135
 MBSE 2024 XII CHEMISTRY SOLUTIONS | Instagram – #136
MBSE 2024 XII CHEMISTRY SOLUTIONS | Instagram – #136
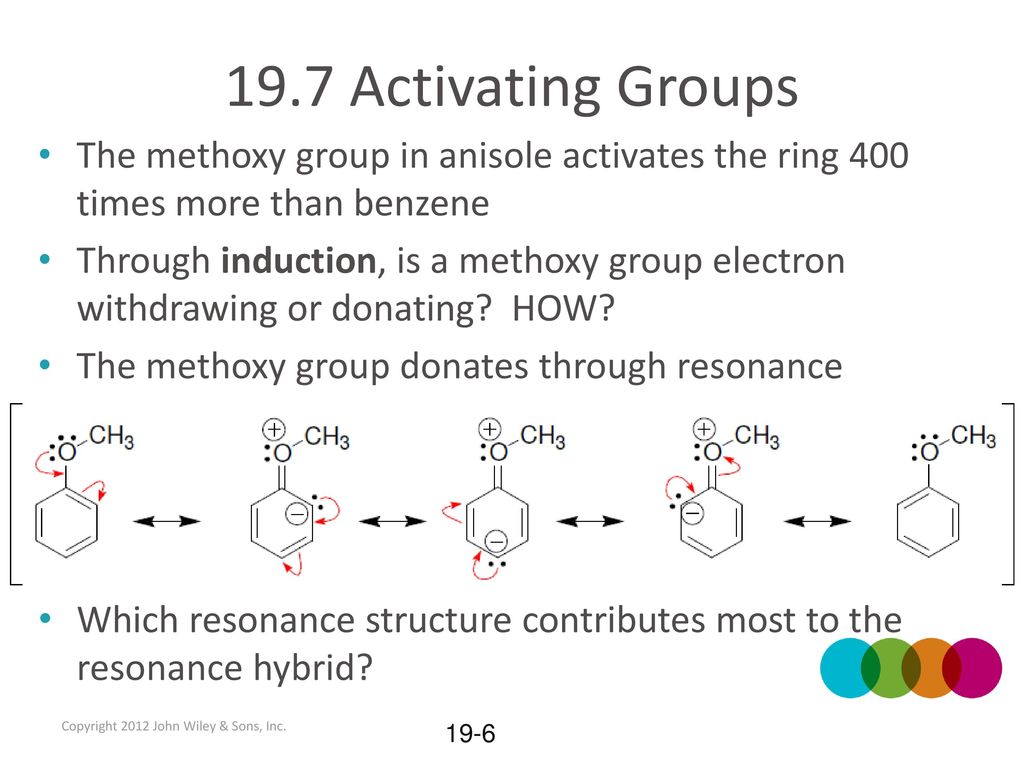 PPT – 275 PowerPoint Presentation, free download – ID:1105001 – #137
PPT – 275 PowerPoint Presentation, free download – ID:1105001 – #137
 is not a correct… – Chemistry Online | Facebook – #138
is not a correct… – Chemistry Online | Facebook – #138
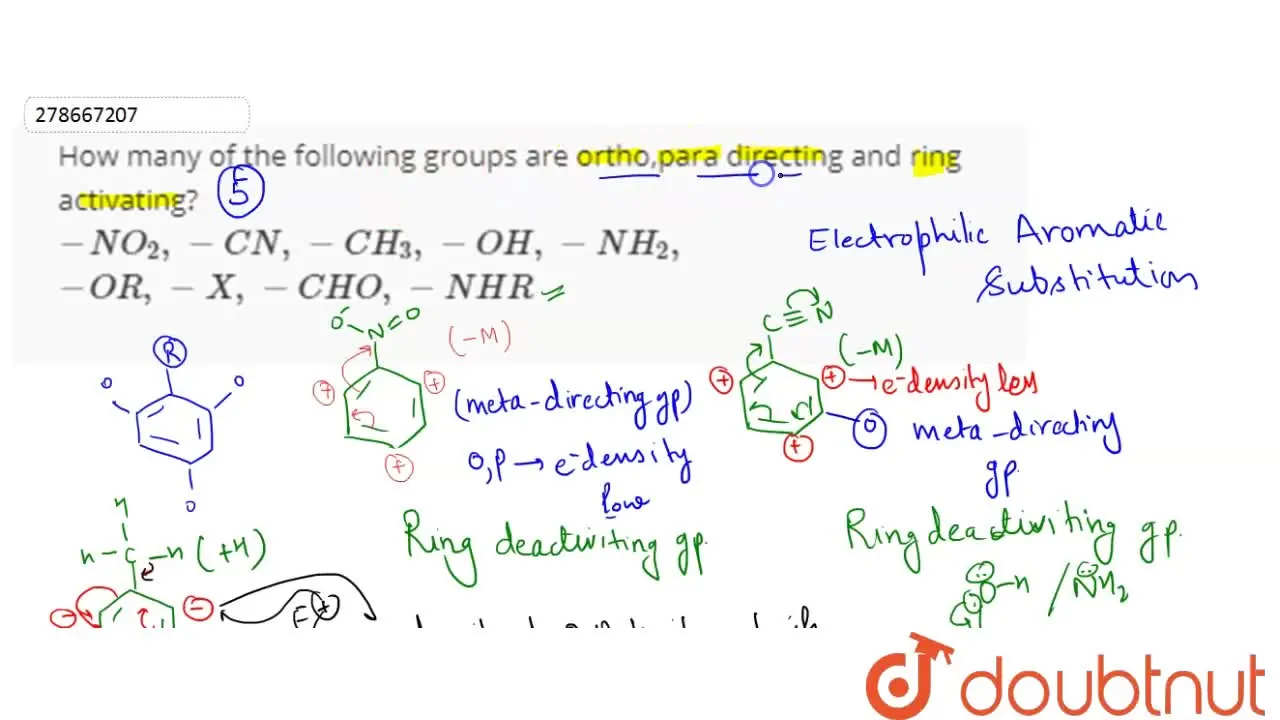
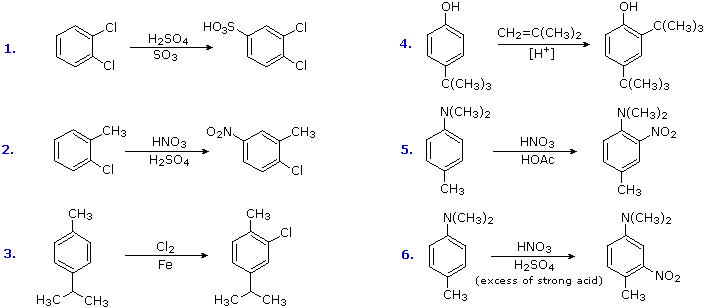 How many of the following groups are ortho,para directing and ring activating? -NO_(2), -CN, -CH… – YouTube – #139
How many of the following groups are ortho,para directing and ring activating? -NO_(2), -CN, -CH… – YouTube – #139
 PPT – 16. Chemistry of Benzene: Electrophilic Aromatic Substitution PowerPoint Presentation – ID:61425 – #140
PPT – 16. Chemistry of Benzene: Electrophilic Aromatic Substitution PowerPoint Presentation – ID:61425 – #140
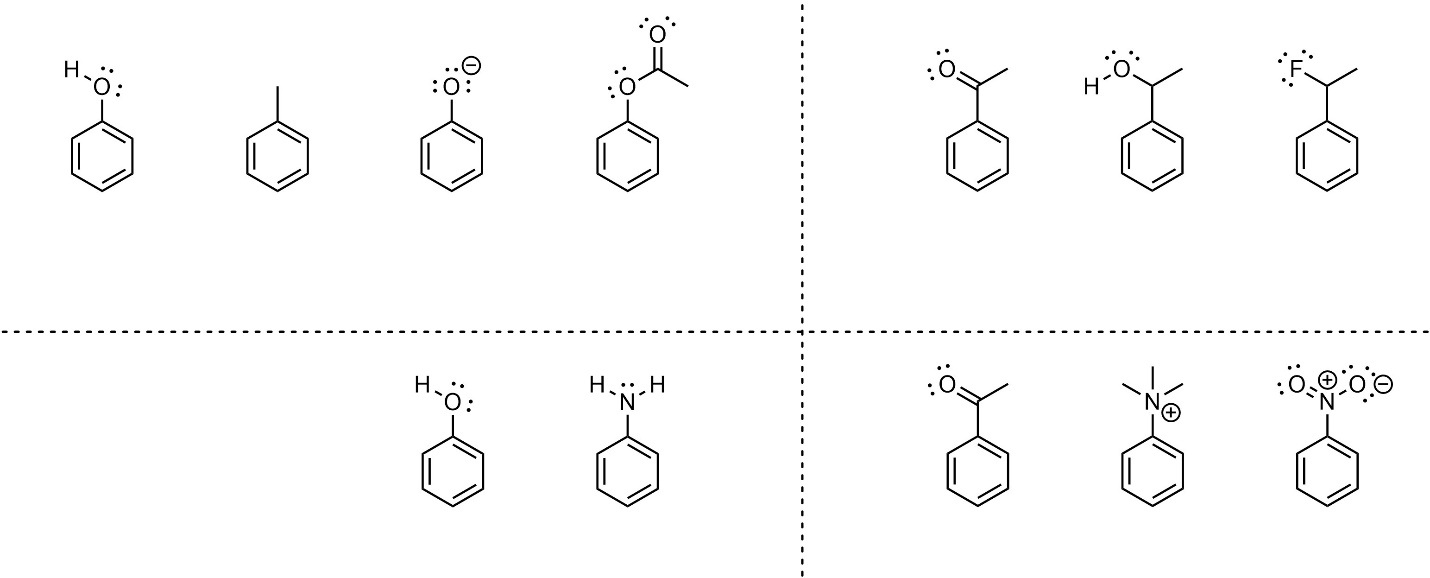 Electrophilic Aromatic Substitution Rxns Practice Exam | PDF | Organic Chemistry | Chemistry – #141
Electrophilic Aromatic Substitution Rxns Practice Exam | PDF | Organic Chemistry | Chemistry – #141
 Organic Chemistry 2015-2016 B.Sc Chemistry Semester 5 (TYBSc) 2013 Pattern question paper with PDF download | Shaalaa.com – #142
Organic Chemistry 2015-2016 B.Sc Chemistry Semester 5 (TYBSc) 2013 Pattern question paper with PDF download | Shaalaa.com – #142
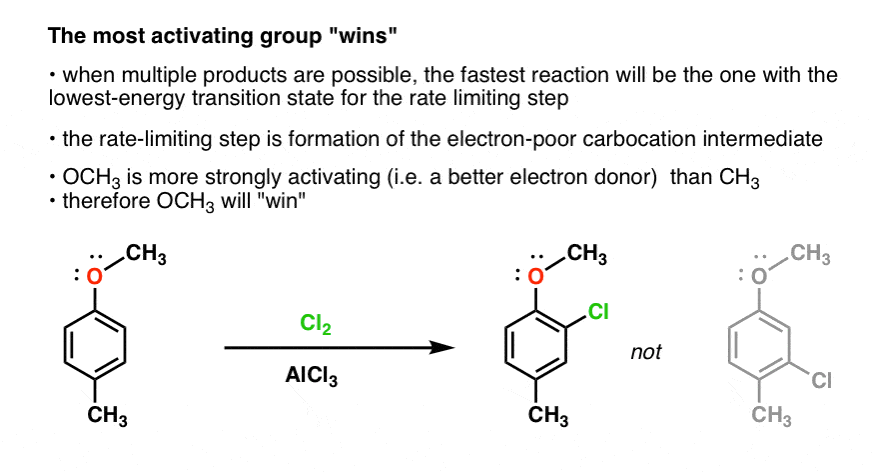
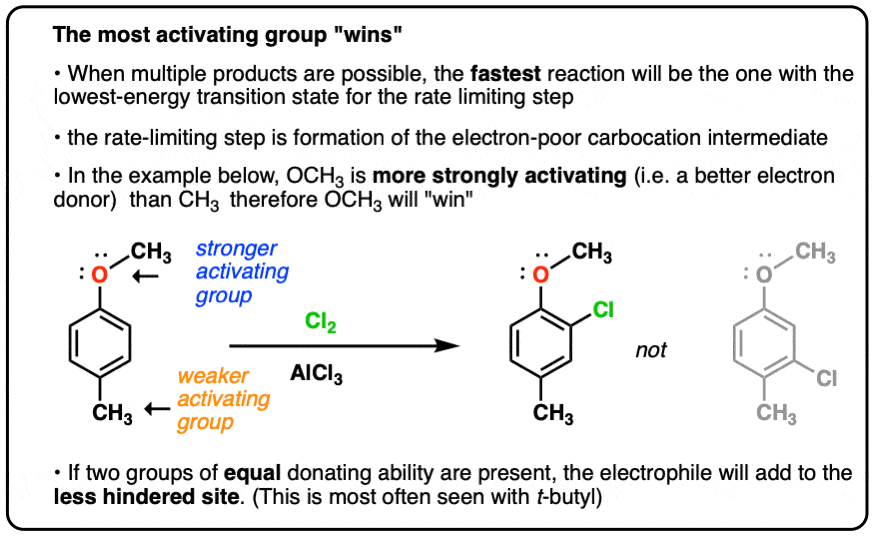
 EAS On Disubstituted Benzenes: The Strongest Electron-Donor “Wins” – #143
EAS On Disubstituted Benzenes: The Strongest Electron-Donor “Wins” – #143
 Activating ortho pera directing group which contain unshare pair of Electron |Orientation in benzene – YouTube – #144
Activating ortho pera directing group which contain unshare pair of Electron |Orientation in benzene – YouTube – #144
- electron withdrawing groups resonance
- electron withdrawing groups acidity
- meta directing groups examples
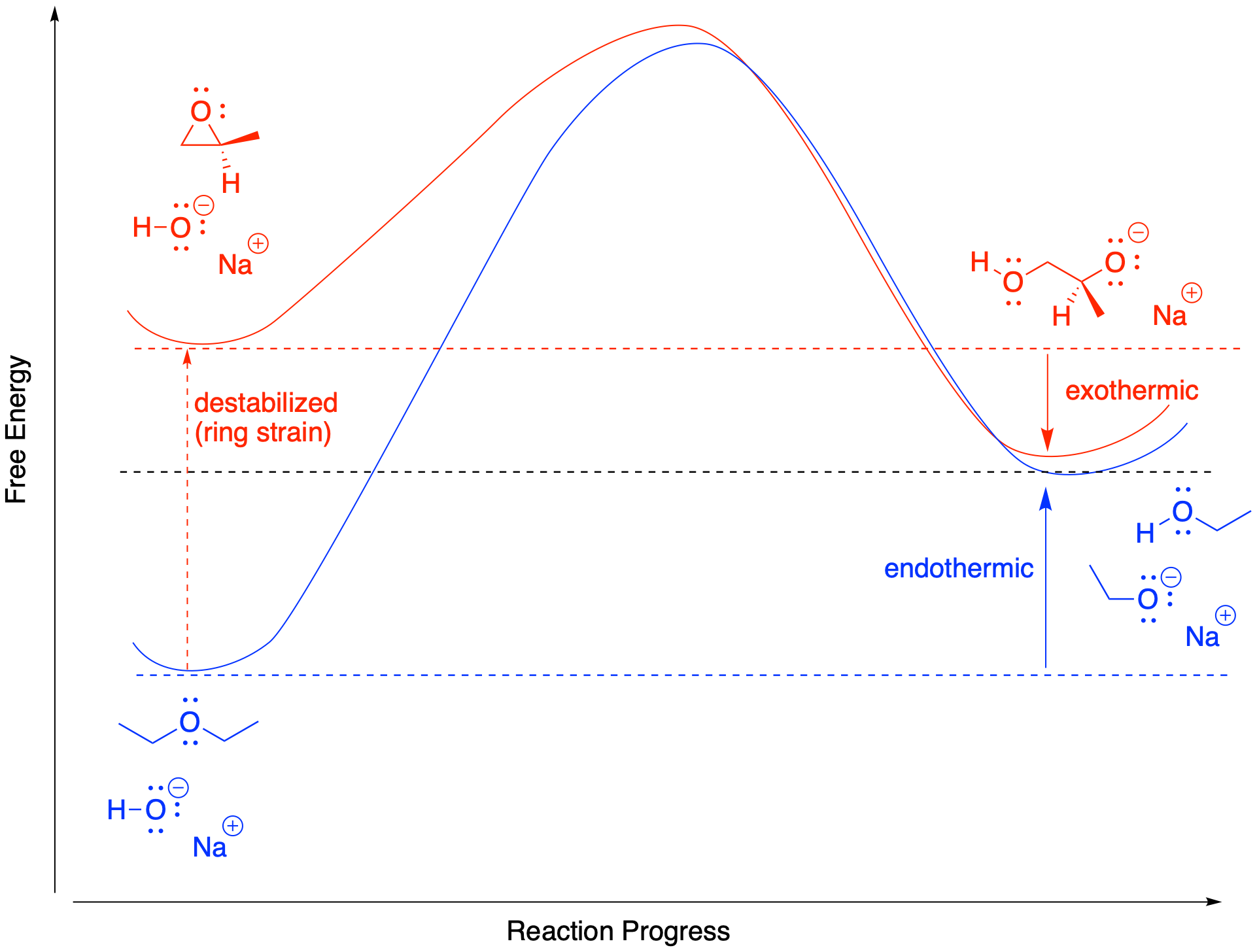 Answered: A F A CH₂CH3 CH3 B CF3 B CH3 Reaction:… | bartleby – #145
Answered: A F A CH₂CH3 CH3 B CF3 B CH3 Reaction:… | bartleby – #145
 Halogenation of Benzene – Chemistry Steps – #146
Halogenation of Benzene – Chemistry Steps – #146
 SOLVED: In electrophilic aromatic substitution reactions, the -CCl3 group is an activating group because it donates electron density through induction deactivating group because it withdraws electron density through resonance C an activating – #147
SOLVED: In electrophilic aromatic substitution reactions, the -CCl3 group is an activating group because it donates electron density through induction deactivating group because it withdraws electron density through resonance C an activating – #147
 Which molecules will undergo aromatic bromination (“Br”_2, “FeBr”_3) the fastest? Why? | Socratic – #148
Which molecules will undergo aromatic bromination (“Br”_2, “FeBr”_3) the fastest? Why? | Socratic – #148
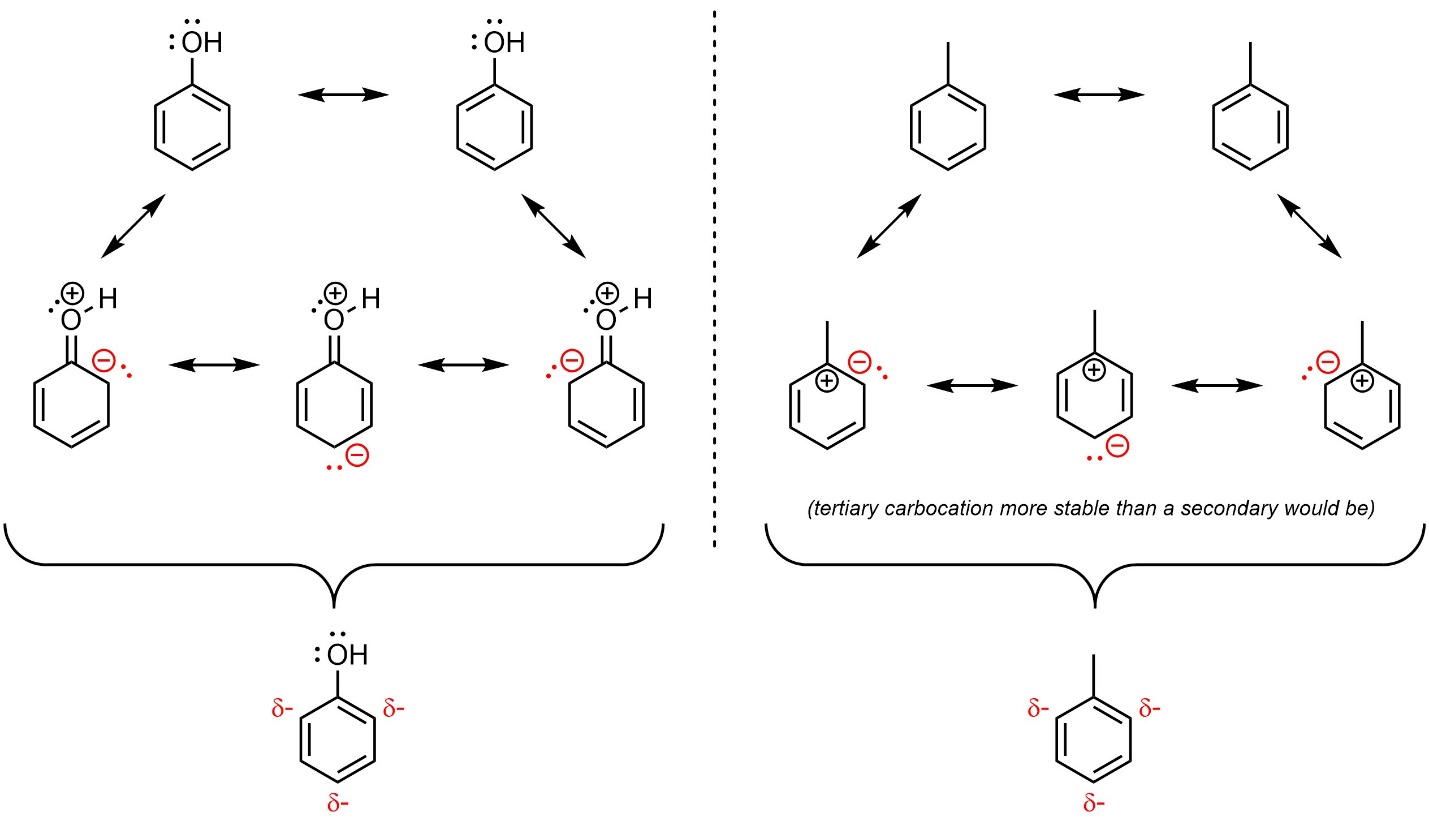 Solved 1. Which of the following statements about | Chegg.com – #149
Solved 1. Which of the following statements about | Chegg.com – #149
 How does the NO2 group deactivate the aromatic ring towards electrophilic substitution reaction? – Quora – #150
How does the NO2 group deactivate the aromatic ring towards electrophilic substitution reaction? – Quora – #150
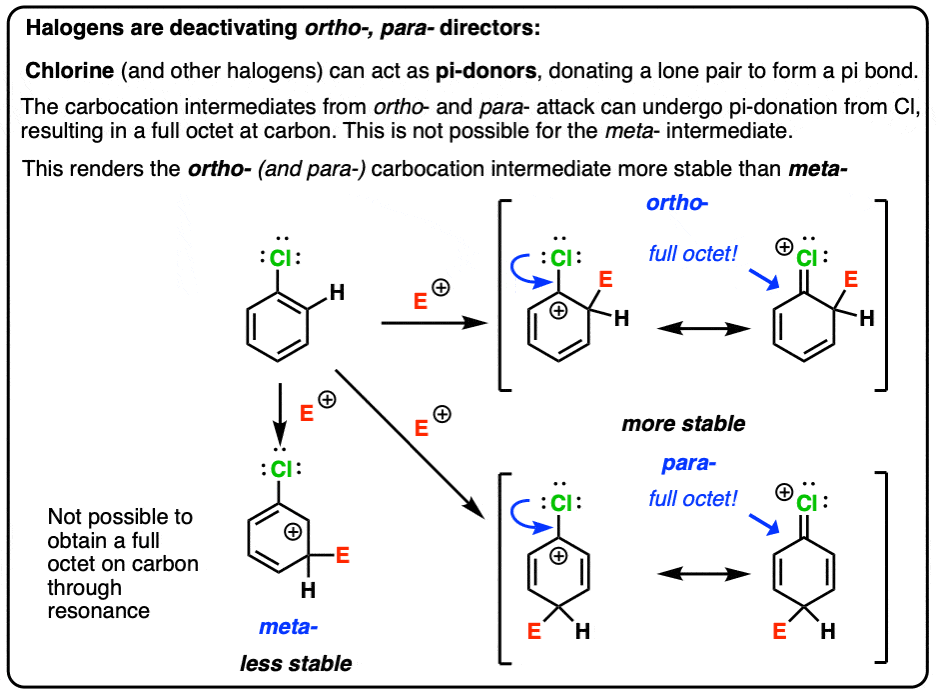
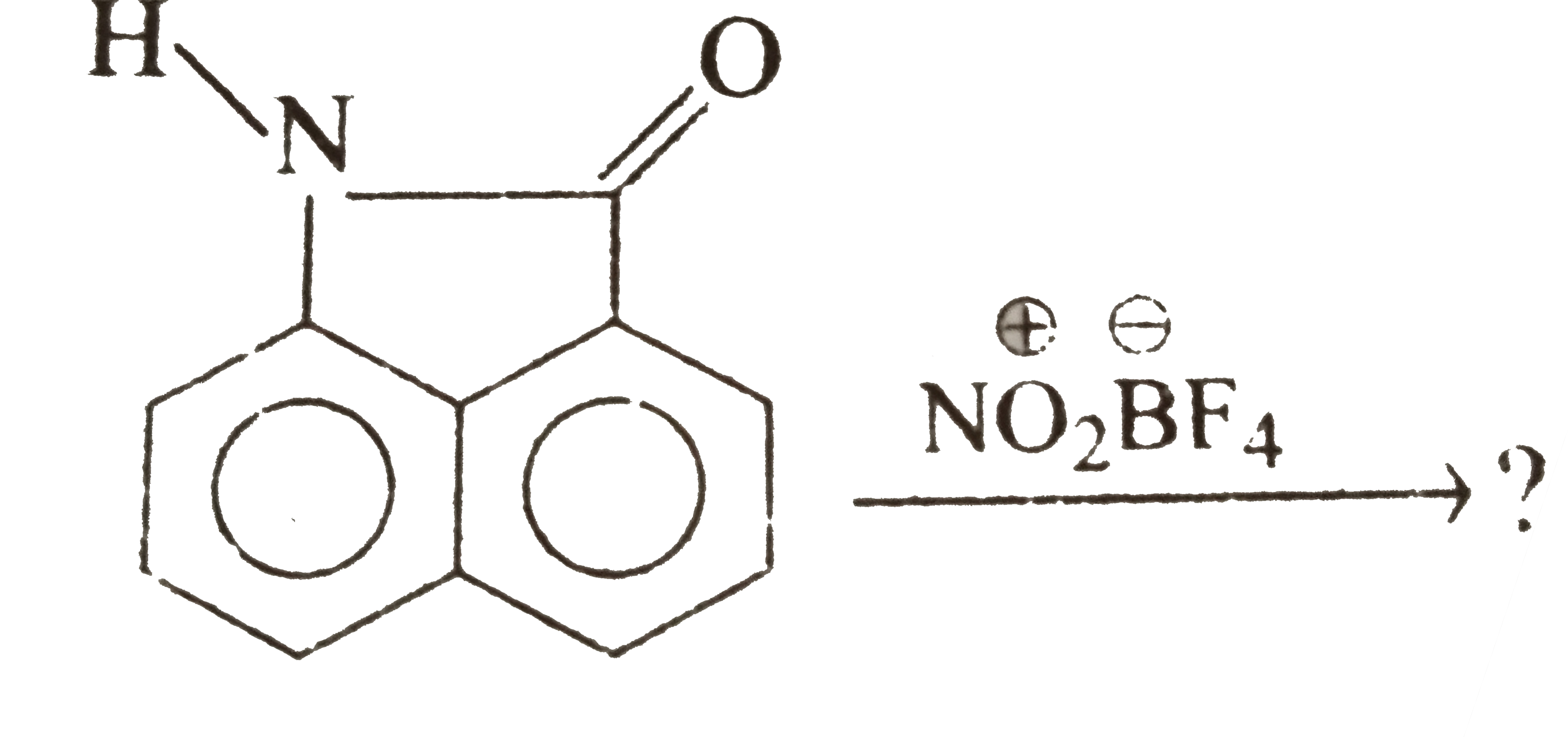 Although chlorine is an electron withdrawing group, yet it is ortho-, para- directing in electrophilic aromatic substitution reaction. Explain why it is so? – #151
Although chlorine is an electron withdrawing group, yet it is ortho-, para- directing in electrophilic aromatic substitution reaction. Explain why it is so? – #151
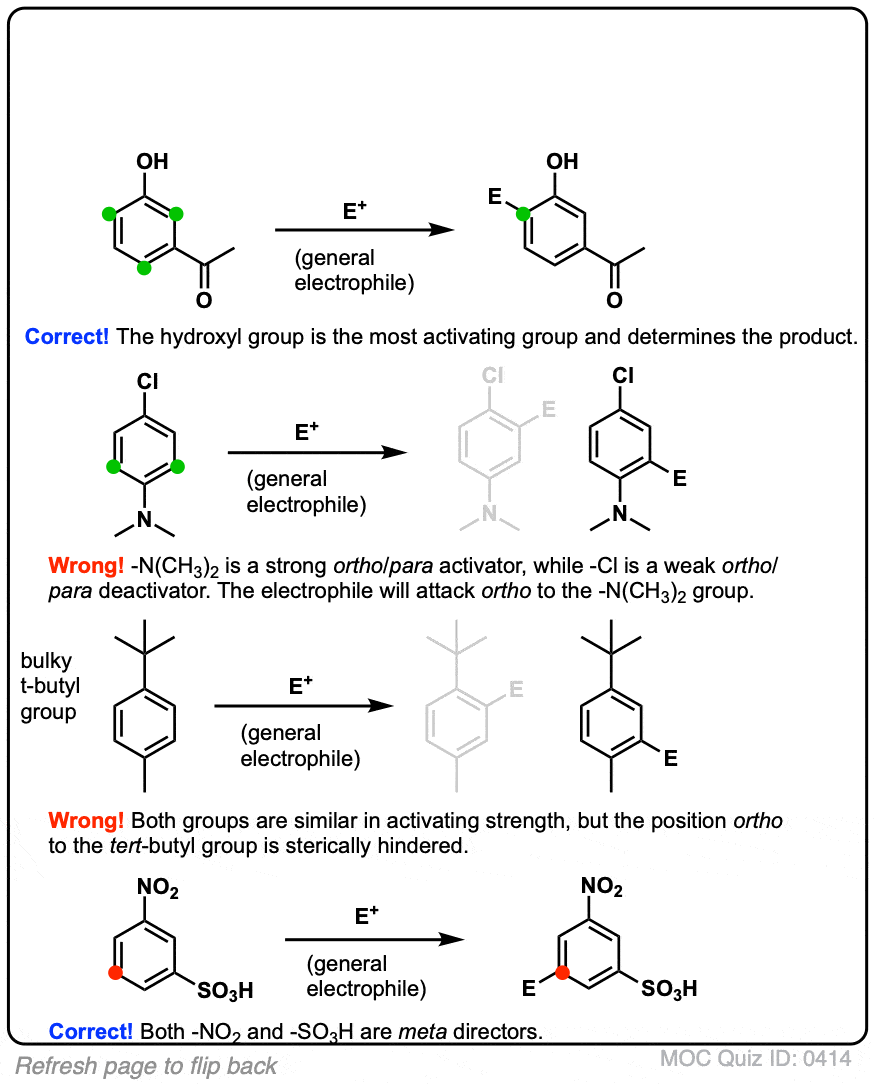 The compound P, Q, and S were separately subjected to nitration using $HN{{O}_{3}}\/{{H}_{2}}S{{O}_{4}}$ mixture. The major product formed in each case respectively is:\n \n \n \n \n \n \n \n \n \n – #152
The compound P, Q, and S were separately subjected to nitration using $HN{{O}_{3}}\/{{H}_{2}}S{{O}_{4}}$ mixture. The major product formed in each case respectively is:\n \n \n \n \n \n \n \n \n \n – #152
 Toluene, when treated with $ B{{r}_{2}}\/Fe $ gives p-bromotoluene as the major product because the $ C{{H}_{3}} $ group:(A) Is para directing.(B) Is Meta directing.(C) Activates the ring by hyperconjugation.(D) Deactivates the – #153
Toluene, when treated with $ B{{r}_{2}}\/Fe $ gives p-bromotoluene as the major product because the $ C{{H}_{3}} $ group:(A) Is para directing.(B) Is Meta directing.(C) Activates the ring by hyperconjugation.(D) Deactivates the – #153
 Genes | Free Full-Text | Epigenetic Alterations in Sports-Related Injuries – #154
Genes | Free Full-Text | Epigenetic Alterations in Sports-Related Injuries – #154
 A-Level Chemistry: Benzene and its compounds Part 15 – YouTube – #155
A-Level Chemistry: Benzene and its compounds Part 15 – YouTube – #155
 Substitution Reactions of Benzene Derivatives – Chemistry LibreTexts – #156
Substitution Reactions of Benzene Derivatives – Chemistry LibreTexts – #156
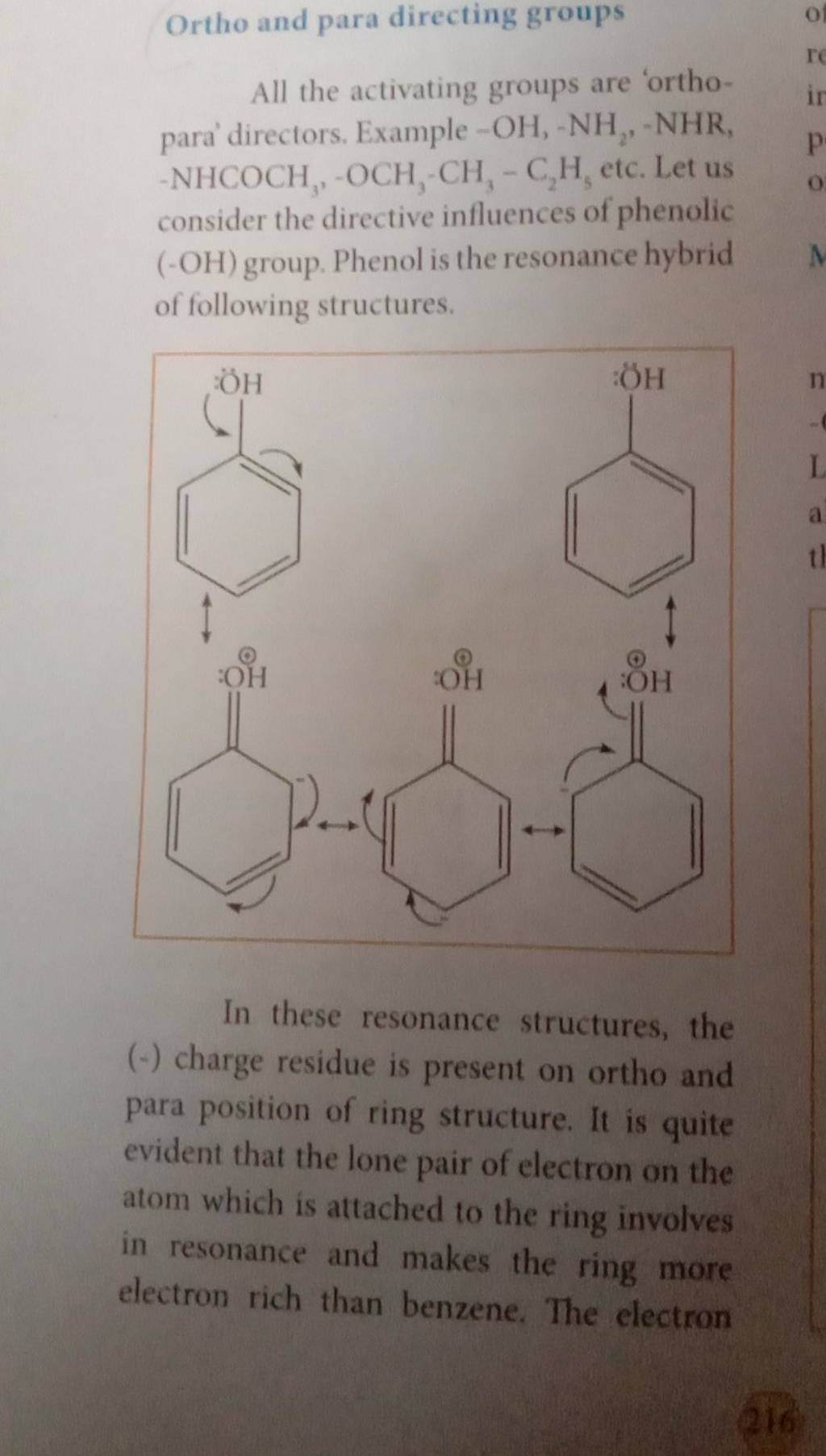
 Ortho and para directing groups All the activating groups are ‘orthopara’.. – #157
Ortho and para directing groups All the activating groups are ‘orthopara’.. – #157
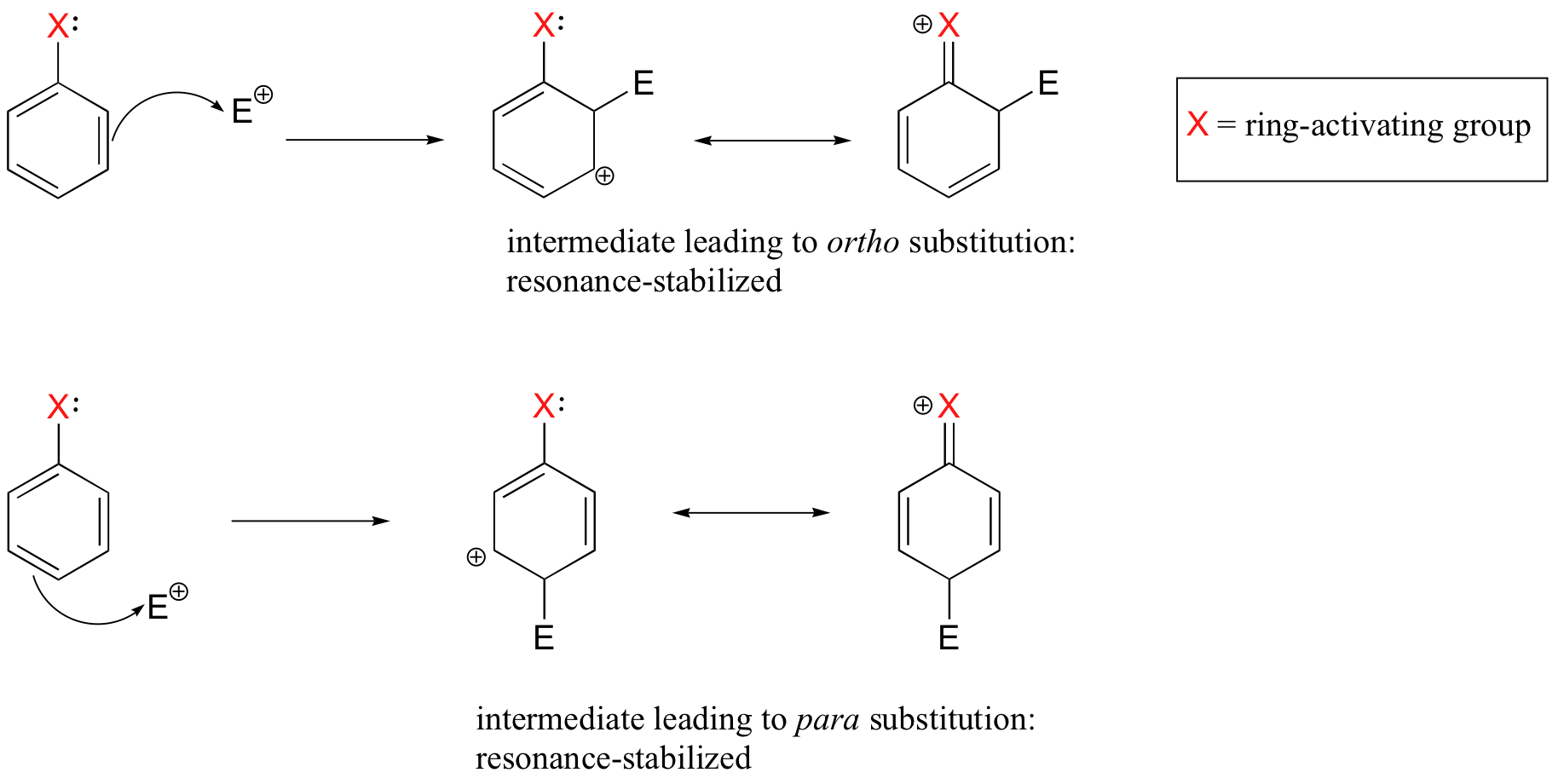
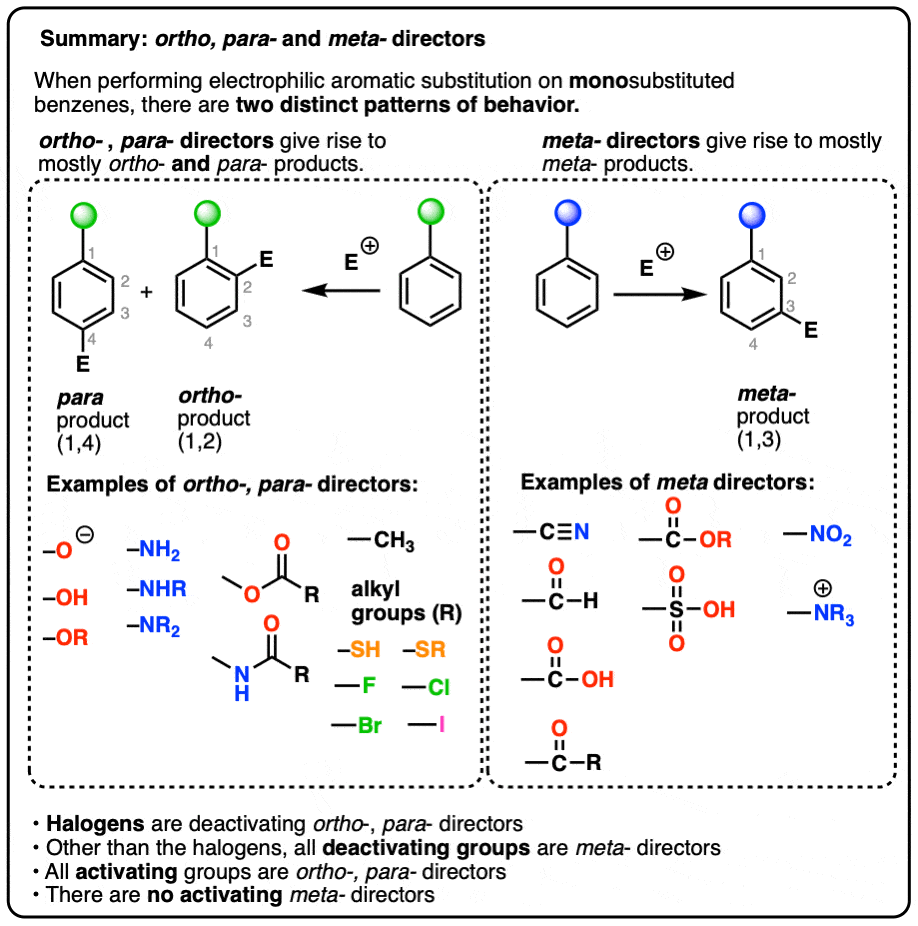
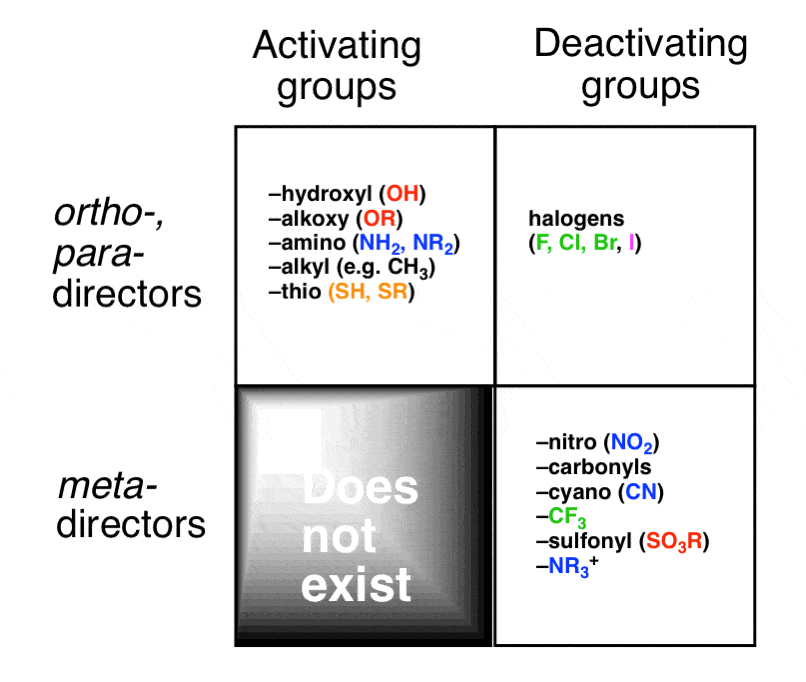
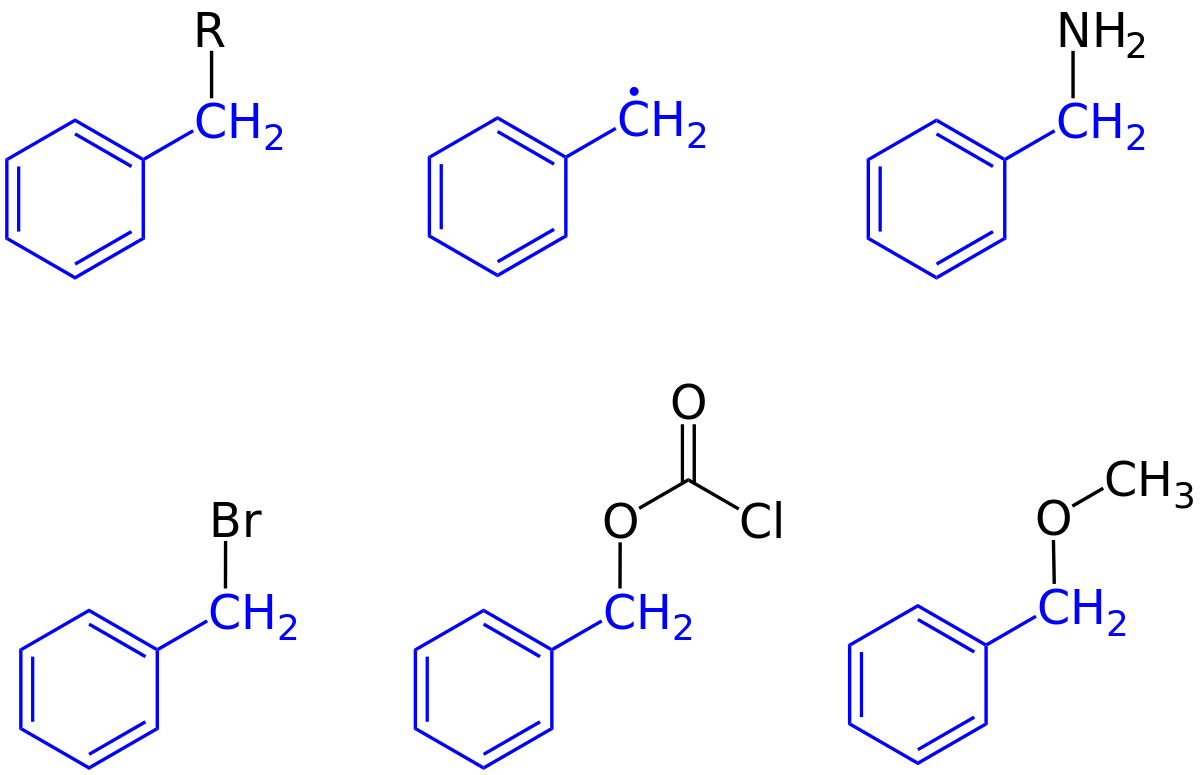 Ortho-, Para- and Meta- Directors in Electrophilic Aromatic Substitution – #158
Ortho-, Para- and Meta- Directors in Electrophilic Aromatic Substitution – #158
 Organic Chemistry for A-Level: Benzene and Its Derivates – #159
Organic Chemistry for A-Level: Benzene and Its Derivates – #159
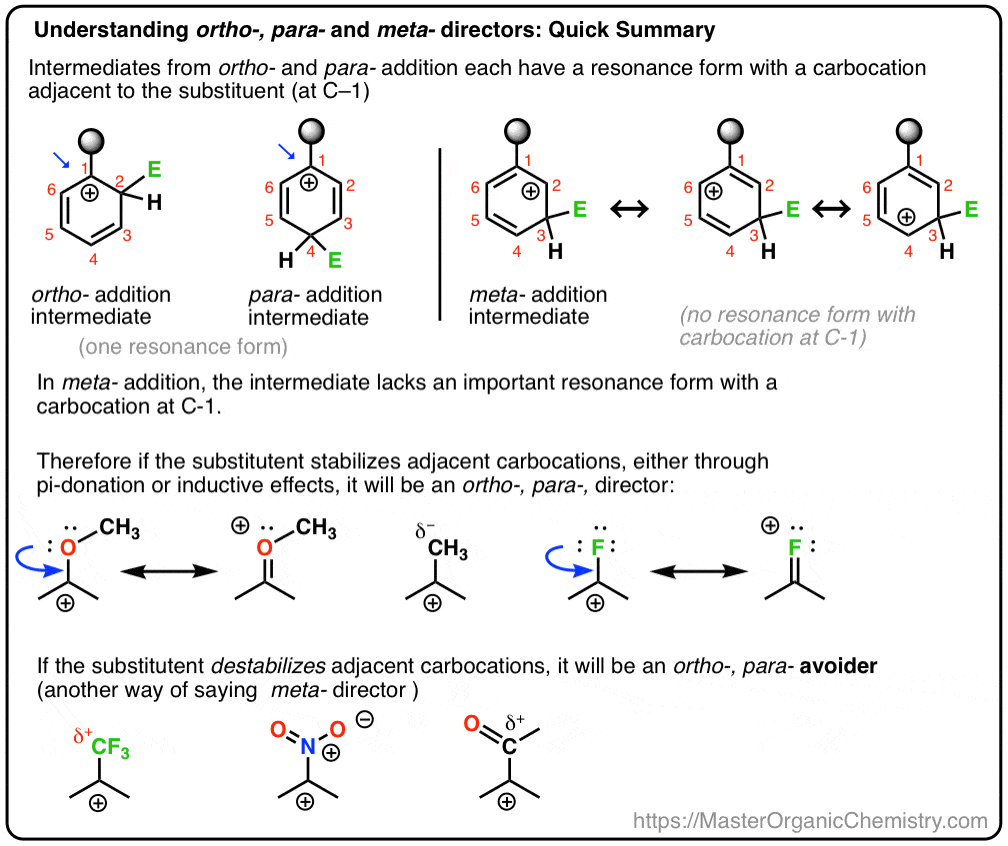 Activating and Deactivating groups; | by Farwa Shah | Medium – #160
Activating and Deactivating groups; | by Farwa Shah | Medium – #160
Posts: ring activating groups
Categories: Rings
Author: dienmayquynhon.com.vn
